Quality control method of cefixime
A quality control method and technology of cefixime, applied in measuring devices, instruments, scientific instruments, etc., can solve the problem of poor separation degree and/or peak shape of impurities, poor comprehensive detection effect, and poor detection effect of special unknown impurities And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
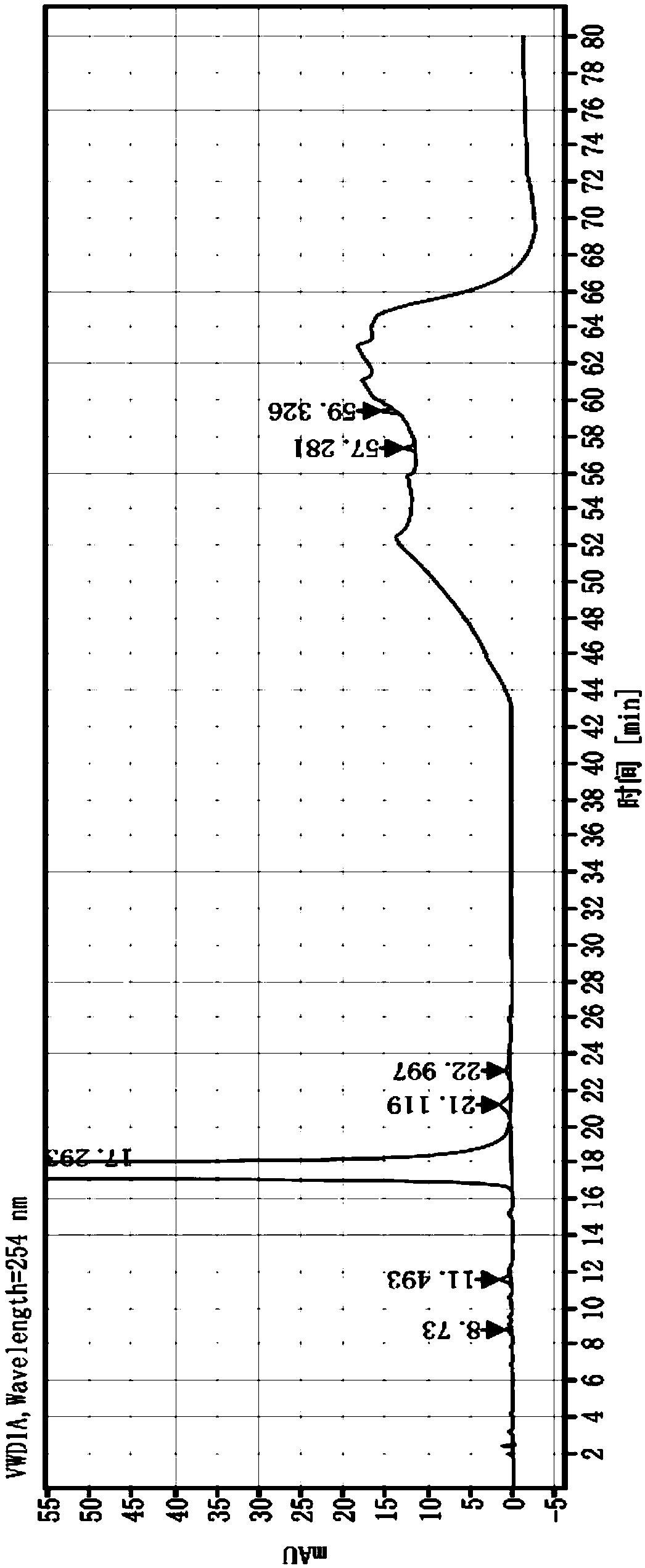
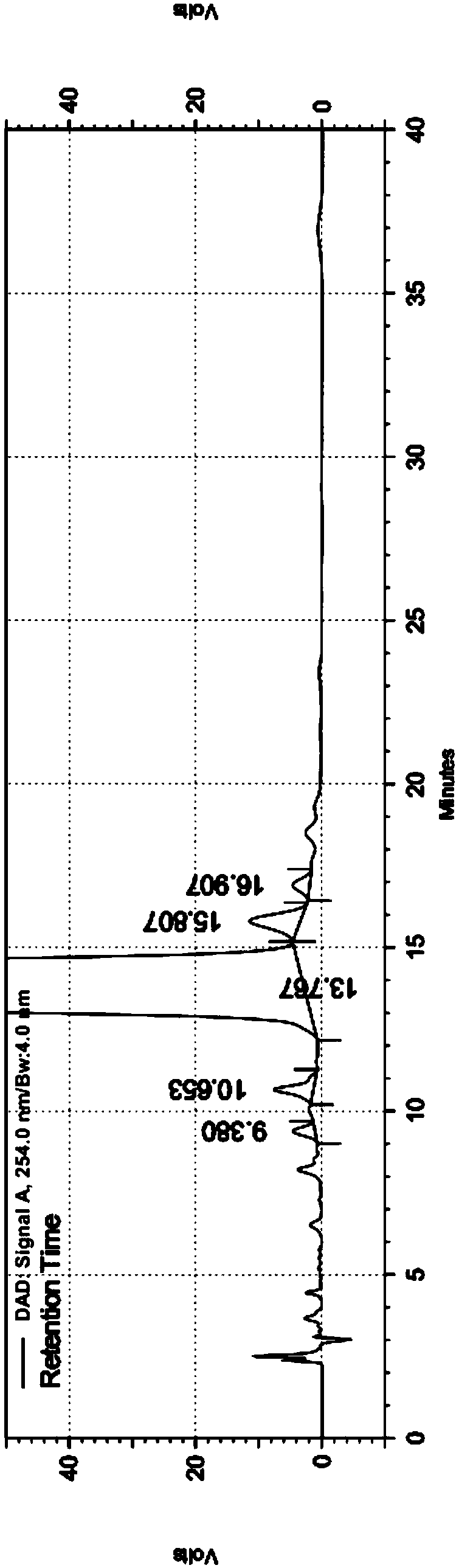
Embodiment 1
[0049] Chromatographic column: Agilent ZORBAX SB-C18, 250×4.6mm, 5μm
[0050] Mobile phase:
[0051] Phase A: Tetrabutylammonium hydroxide aqueous solution: Acetonitrile = 50:50
[0052] Phase B: Tetrabutylammonium hydroxide aqueous solution: Acetonitrile = 85:15
[0053] Column temperature: 40℃
[0054] Injection volume: 20μL
[0055] Injection concentration 1mg / mL
[0056] Flow rate: 1.2mL / min
[0057] Detection wavelength: 254nm
[0058] The quality control method of described cefixime comprises the following steps:
[0059] 1. Prepare the solution;
[0060] Take 25mL of 10% tetrabutylammonium hydroxide solution, add purified water to dilute to 1000mL, shake well, adjust pH to 6.5 with 1.5mol / L phosphoric acid solution to obtain tetrabutylammonium hydroxide aqueous solution; mix the corresponding amount of tetrabutylammonium hydroxide aqueous solution with acetonitrile , to obtain A phase and B phase, respectively;
[0061] Take 5 cefixime dispersible tablets (50mg s...
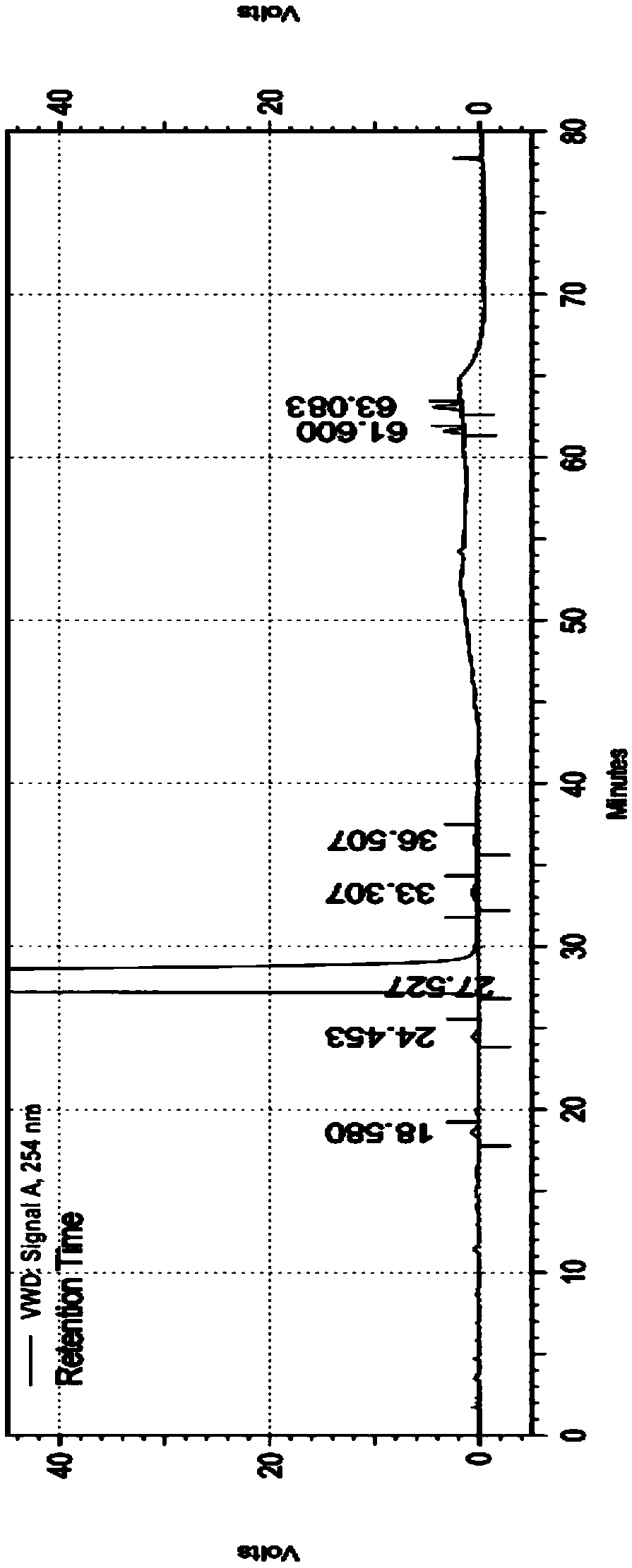
Embodiment 2
[0068] Chromatographic column: Agilent ZORBAX SB-C18, 250×4.6mm, 5μm
[0069] Mobile phase:
[0070] Phase A: Tetrabutylammonium hydroxide aqueous solution: Acetonitrile = 50:50
[0071] Phase B: Tetrabutylammonium hydroxide aqueous solution: Acetonitrile = 85:15
[0072] Column temperature: 40℃
[0073] Injection volume: 20μL
[0074] Injection concentration 1mg / mL
[0075] Flow rate: 1.2mL / min
[0076] Detection wavelength: 254nm
[0077] The quality control method of described cefixime comprises the following steps:
[0078] 1. Prepare the solution;
[0079] Take 25mL of 10% tetrabutylammonium hydroxide solution, add purified water to dilute to 1000mL, shake well, adjust pH to 6.5 with 1.5mol / L phosphoric acid solution to obtain tetrabutylammonium hydroxide aqueous solution; mix the corresponding amount of tetrabutylammonium hydroxide aqueous solution with acetonitrile , to obtain A phase and B phase, respectively;
[0080] Take 5 cefixime dispersible tablets (50mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com