Relapse and drug resistance gene mutation detection kit and application method of acute lymphoblastic leukemia
An acute lymphocyte detection kit technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve problems affecting clinical prognosis, promote disease recurrence, etc., achieve strong applicability, reduce false positives, and detect Clear and clear results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Composition and preparation of embodiment 1 kit
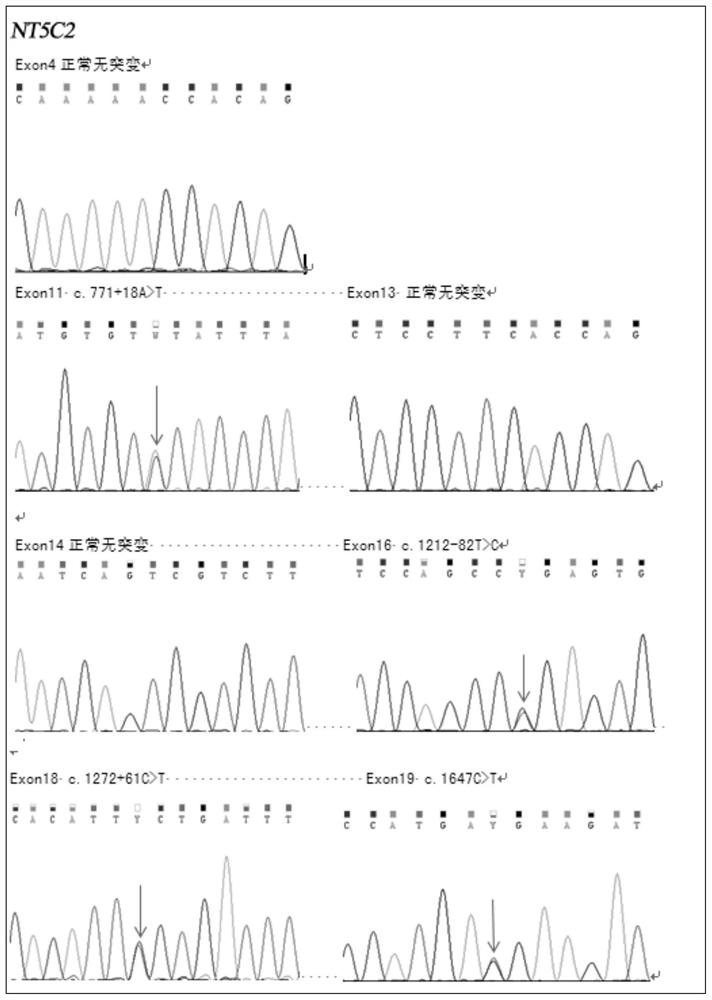
[0046] In the present invention, for acute lymphoblastic leukemia chemotherapy resistance and relapse-related NT5C2 drug resistance gene, a detection kit and application method for acute lymphoblastic leukemia treatment medication and close detection of relapse are developed. This gene is currently the most promising target for the detection of chemotherapy resistance and relapse of acute lymphoblastic leukemia, and can play an important role in the current and future treatment of acute lymphoblastic leukemia.
[0047] The source of the gene sequence detected by the present invention is the NCBI (National Center for Biotechnology Information) gene bank, and the specific identification and functions are shown in Table 1 below:
[0048] Table 1
[0049]
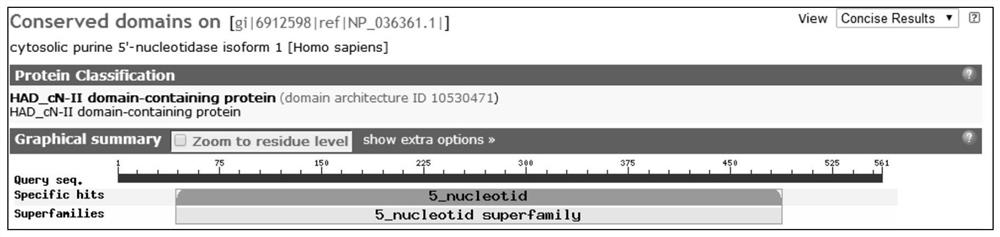
[0050] The domain distribution diagram of the gene-encoded protein detected by the present invention, such as figure 1 shown.
[0051] The invention provides a kit ...
Embodiment 2
[0061] Example 2 Detection and verification report of the kit
[0062] 1. Name of the test procedure (method) of the acute lymphoblastic leukemia drug detection and recurrence monitoring kit of the present invention: NT5C2 gene mutation detection (PCR-generation sequencing method).
[0063] 2. The purpose of verification: through the analysis and evaluation of the accuracy, sensitivity, and precision of the methodology, the method verification of the testing procedure (methodology) is carried out to meet the needs of clinical experiments.
[0064] 3. Experimental materials:
[0065] 3.1. Specimen type: DNA extracted from peripheral blood.
[0066] 3.2. Reagents: This verification experiment uses the conventional reagents of the molecular platform, and all reagents are within the validity period. The main reagent information is shown in Table 4:
[0067] Table 4 Reagent information used for NT5C2-related gene mutation detection verification
[0068]
[0069] 3.3. Instrume...
Embodiment approach
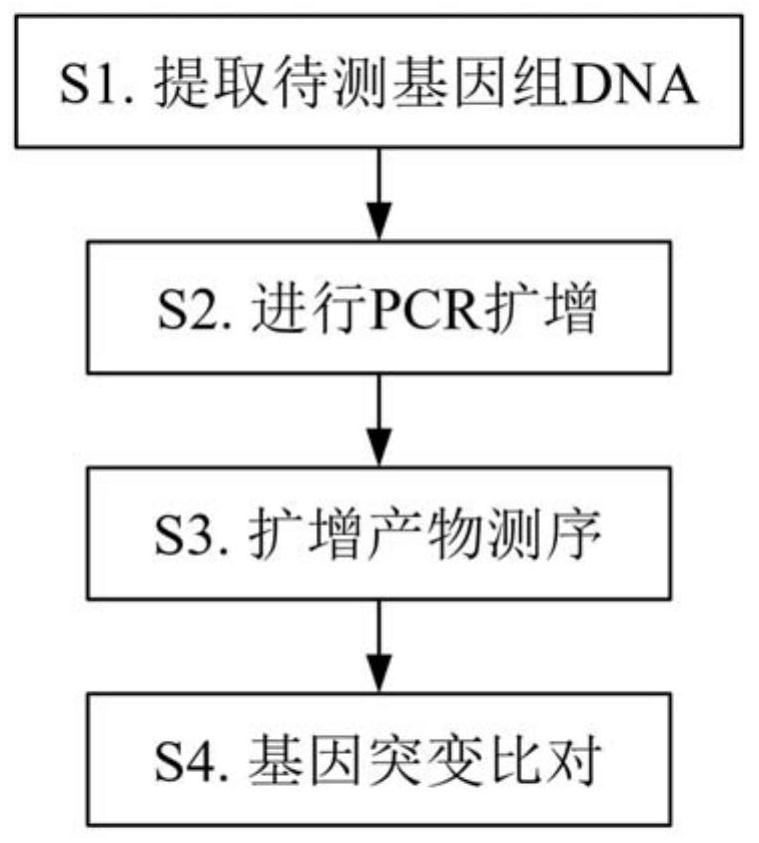
[0082] The specific implementation is as follows:
[0083] In step S1, peripheral blood is used as the sample to be tested. Genomic DNA can be extracted using a conventional kit column extraction method, and operated according to the instructions of the kit used to collect 50 μL of DNA solution with a concentration of 50-200 ng / μL, which can be directly used Before testing or store at -20°C;
[0084] The verification experiment used 7 specimens, and the specimen type was DNA extracted from peripheral blood. See Table 5 for the specimens.
[0085] Table 5. Specimen information used for NT5C2-related gene mutation detection verification
[0086] sample name sample type sample number 1 DNA extracted from peripheral blood p16060306 2 DNA extracted from peripheral blood p16060608 3 DNA extracted from peripheral blood p16061215 4 DNA extracted from peripheral blood p16061242 5 DNA extracted from peripheral blood p16061548 6 DN...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com