Preparation method and application of compound salt formed by glucuronic acid or glucurolactone and sophocarpidine and/or oxymatrine
A technology of glucuronic acid and glucuronide is applied in the field of preparation of double salts, can solve problems such as reduction, and achieve the effects of simple preparation method, reduced dosage of medicines, and reduced stimulation of medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of preparation method of the double salt that is formed by glucuronic acid and matrine as follows, concrete steps are as follows:
[0030] (1) Add 5 g of a mixture of glucuronic acid and matrine in an equimolar ratio to a 100 ml reaction flask, then add 50 ml of methanol, stir and reflux in a water bath at 20° C. until clarification;
[0031] (2) evaporating and concentrating the clear liquid obtained in step (1) at 40°C;
[0032] (3) cooling the concentrated product, refrigerating and crystallizing for 12-24 hours, filtering, washing, and vacuum drying;
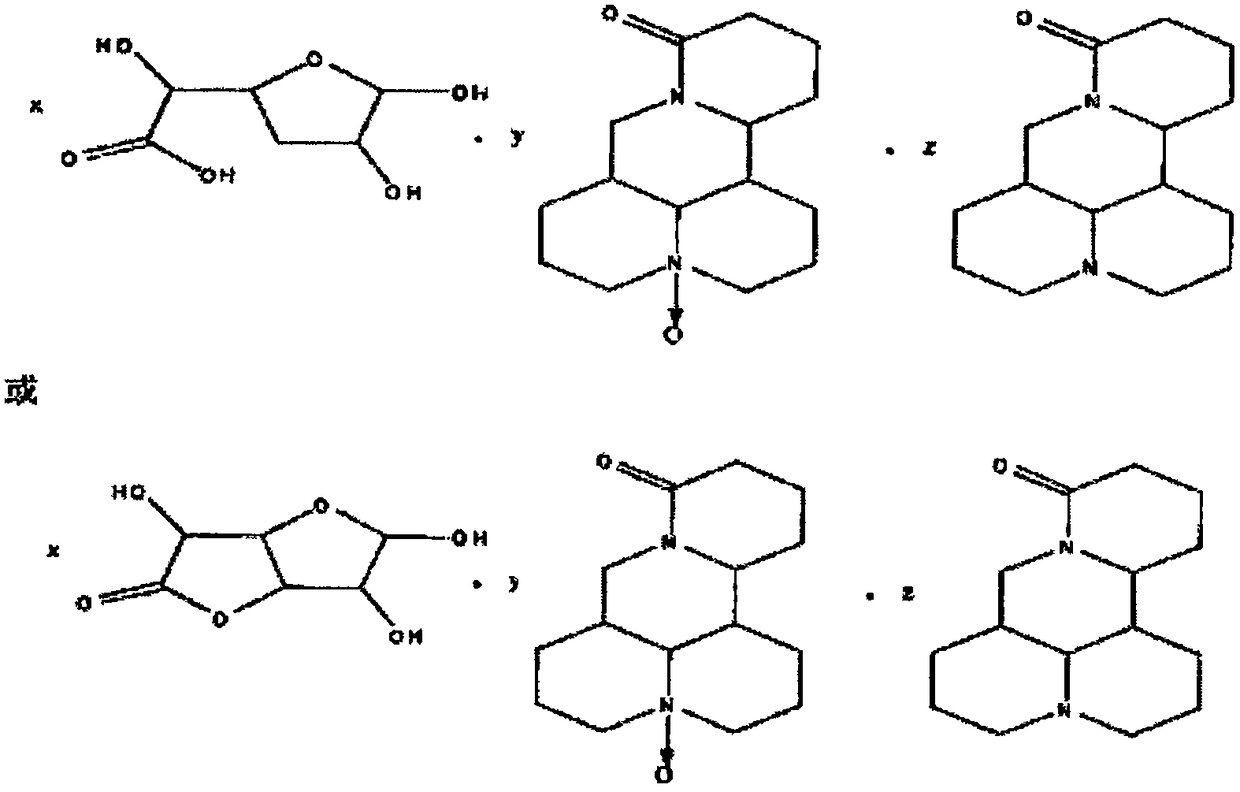
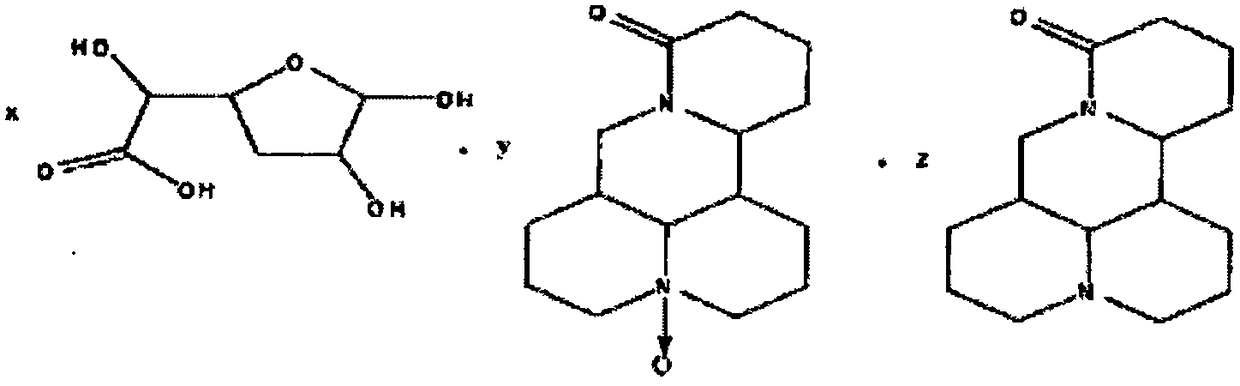
[0033]
[0034] where x=1, y=1, z=0.
Embodiment 2
[0036] A kind of preparation method of the double salt that following formula is formed by glucuronic acid and matrine, concrete steps are as follows:
[0037] (1) Add 5 g of a mixture of glucuronic acid and matrine in an equimolar ratio to a 100 ml reaction flask, then add 50 ml of acetone, stir and reflux in a water bath at 40 ° C until clarification;
[0038] (2) evaporating and concentrating the clear liquid obtained in step (1) at 60°C;
[0039] (3) cooling the concentrated product, refrigerating and crystallizing for 12-24 hours, filtering, washing, and vacuum drying;
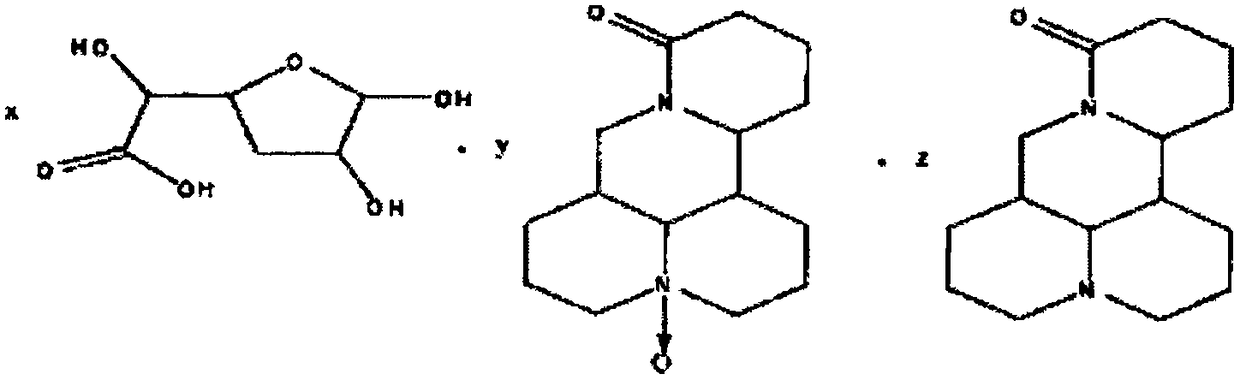
[0040]
[0041] where x=1, y=0, z=1.
Embodiment 3
[0043] A kind of preparation method of the double salt that following formula is formed by glucuronic acid, matrine and matrine, concrete steps are as follows:
[0044] (1) Add 5 g of a mixture of glucuronic acid, matrine and matrine in an equimolar ratio to a 100 ml reaction bottle, then add 50 ml of ethanol, stir and reflux in a water bath at 60 ° C until clarification;
[0045] (2) evaporating and concentrating the clear liquid obtained in step (1) at 80°C;
[0046] (3) cooling the concentrated product, refrigerating and crystallizing for 12-24 hours, filtering, washing, and vacuum drying;
[0047]
[0048] where x=1, y=1, z=1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com