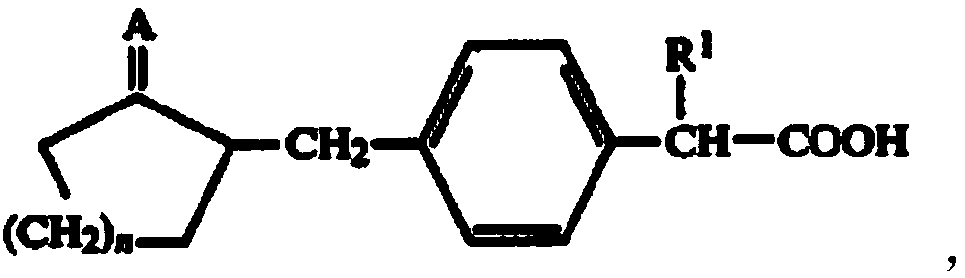
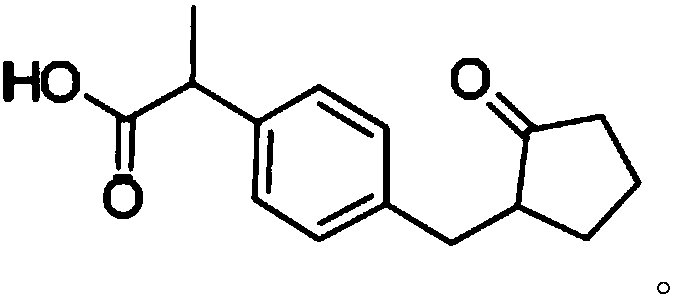
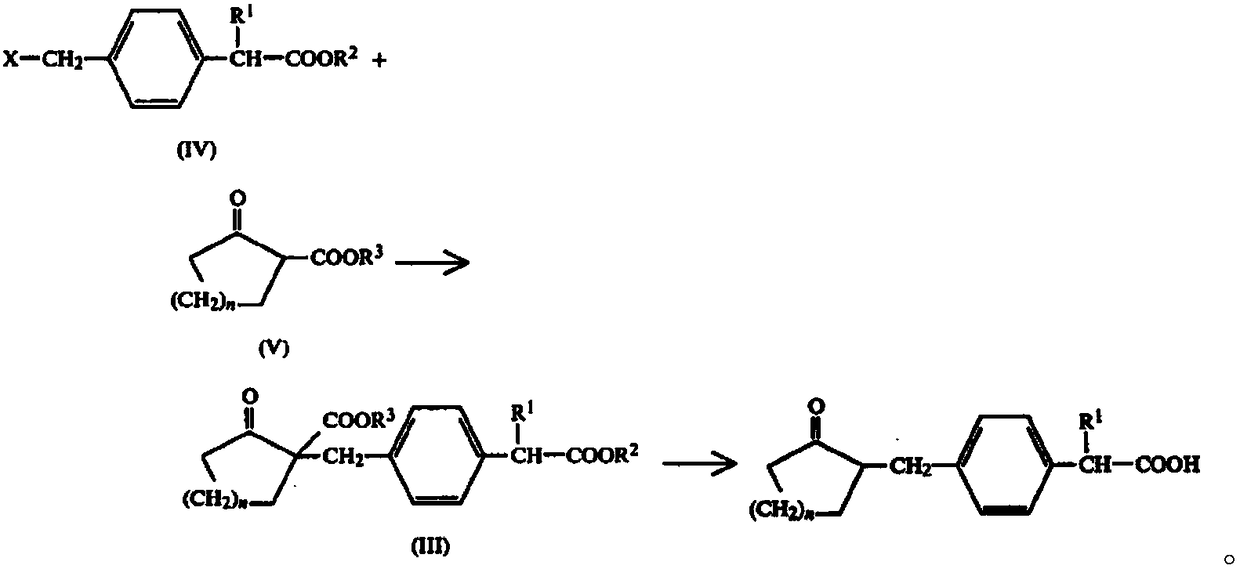
Preparation method of substituted phenylacetic acid derivative
A low-substitution, compound technology, applied in the field of drug synthesis, can solve problems that are not suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] In a 3L reactor, add 1.1kg (7w) of dichloromethane, 333.4g (2.5mol) of anhydrous aluminum trichloride, heat up to reflux, drop adipic anhydride (140.8g dissolved in 470g of dichloromethane, Adipic anhydride is prepared by dehydration of adipic acid), and keep warm for 2-3 hours after the dropwise addition is completed. 157g (1mol) of bromobenzene was added dropwise under reflux, and 3-5 hours after the addition was completed, a sample was taken by HPLC to detect that the raw material was <0.5%, and 160g (5mol) of methanol was added dropwise, kept for 3-4 hours, and the reaction was complete by HPLC detection. Cool to room temperature, add the reaction solution to 1.78kg (10w) of crushed ice, stir well, separate the organic phase, extract the aqueous phase twice with 356g (2w) dichloromethane, combine the organic phases, and use 356g (2w) The 5% sodium bicarbonate solution was washed once, and the organic phase was separated and concentrated to obtain a yellow solid A-5,...
Embodiment 2
[0081] In a 3L reactor, add 1.1kg (7w) of dichloromethane, 333.4g (2.5mol) of anhydrous aluminum trichloride, heat up and reflux, drop 140.8g of adipic anhydride dissolved in 470g of dichloromethane, drop After the addition is complete, keep warm for 2-3 hours. 178g (1mol) of ethyl 2-phenylpropionate was added dropwise under reflux, and 3-5 hours after the addition, HPLC was taken to detect that the raw material was <0.5%, and 160g (5mol) of methanol was added dropwise, kept for 3-4 hours, and detected by HPLC. The response is complete. Cool to room temperature, add the reaction solution to 1.78kg (10w) of crushed ice, stir well, separate the organic phase, extract the aqueous phase twice with 356g (2w) dichloromethane, combine the organic phases, and use 356g (2w) The 5% sodium bicarbonate solution was washed once, and the organic phase was separated and concentrated to obtain a yellow solid A-5, which was beaten with 356g (2w) of n-heptane, filtered with suction, and dried ...
Embodiment 3
[0083] In a 30L dry reaction kettle, add 16.00kg of monomethyl adipate, add 11.30kg (0.95eq) of thionyl chloride dropwise at a temperature of 10-25°C, and finish the dropwise addition in about 5 hours. After the dropwise completion, stir at room temperature After 2 hours, control in ethanol derivatization. After the reaction is completed, concentrate in a rotary evaporator water bath at 25-30°C to obtain an oily substance, which is dried with 5kg of dichloromethane to obtain 16.02kg of compound A-4. Yield 90.0%, GC 92.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com