Method for preparing 3-acetyl ginsenoside F1
A technology of acetyl human and ginsenoside, applied in the field of chemistry, can solve the problems of weak tyrosinase inhibitory activity, unsuitable for large-scale preparation, strong dead adsorption, etc., and achieves the effect of easy large-scale application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
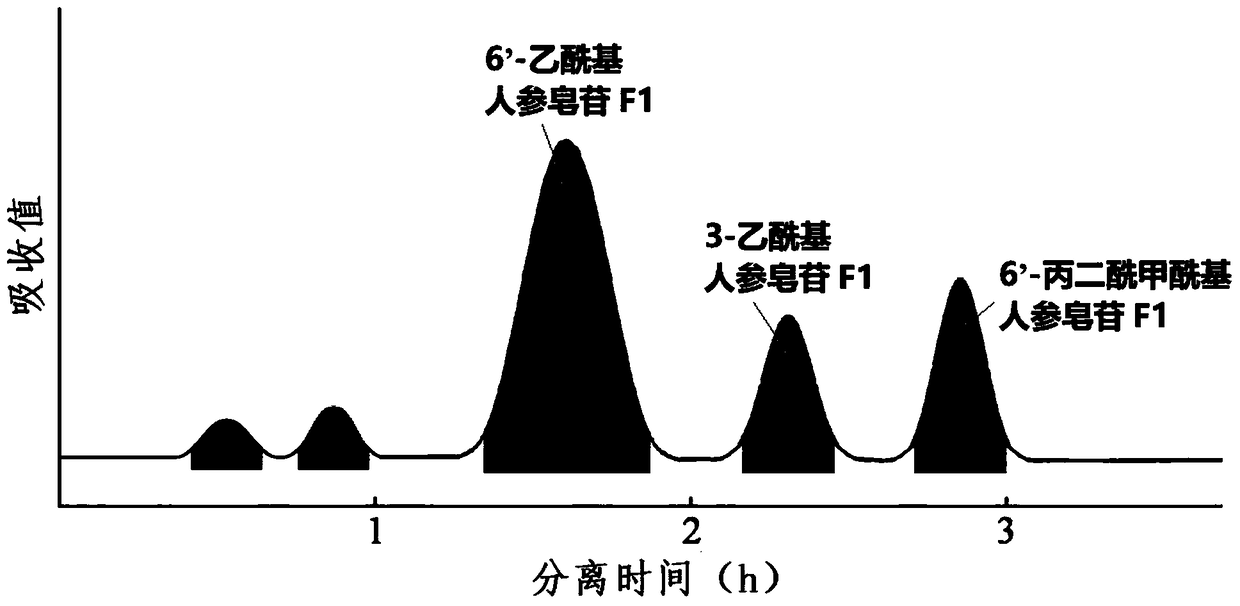
Image
Examples
Embodiment Construction
[0028] The following describes the substantive content of the present invention in detail in conjunction with the drawings and embodiments, but does not limit the protection scope of the present invention.
[0029] 1. Experimental materials and instruments
[0030] Dried ginseng buds were purchased from Bozhou Traditional Chinese Medicine Market.
[0031] LX-68 macroporous adsorption resin was purchased from Zhengzhou Qinshi Technology Co., Ltd.
[0032] High-speed countercurrent chromatography TEB300B was purchased from Shanghai Tongtian Biotechnology Co., Ltd.
[0033] 2. Experimental methods and results
[0034] 1. Preparation of the total extract
[0035] Get 5kg of dried ginseng flower buds, pulverize them with a pulverizer, and pass through an 80-mesh sieve to obtain ginseng flower bud powder. Then use 95% ethanol to extract under heat reflux for 3 times at a solid-to-liquid ratio of 1:10, each time for 2 hours, combine the extracts, concentrate them into a paste, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com