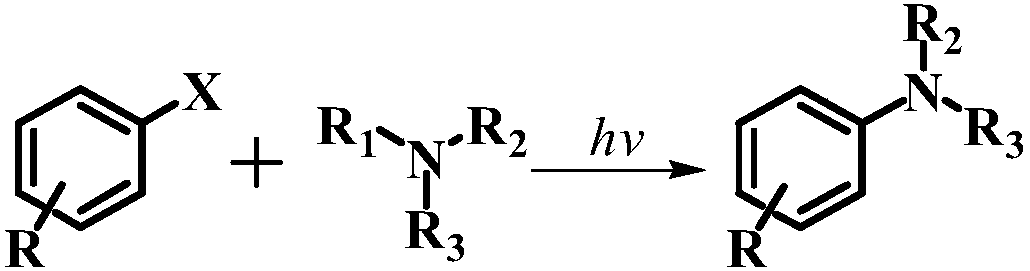Method for preparing secondary aromatic amine or tertiary aromatic amine by conducting amination on aryl halide or alkyl halide
A technology for halogenated alkane amines and halogenated aromatic hydrocarbons is applied in the field of synthesizing aromatic secondary amines or tertiary amines, which can solve the problems of unsuitability for large-scale production, harsh atmosphere requirements and high reaction pressure, avoid side reactions that are difficult to control, realize industrialized production, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
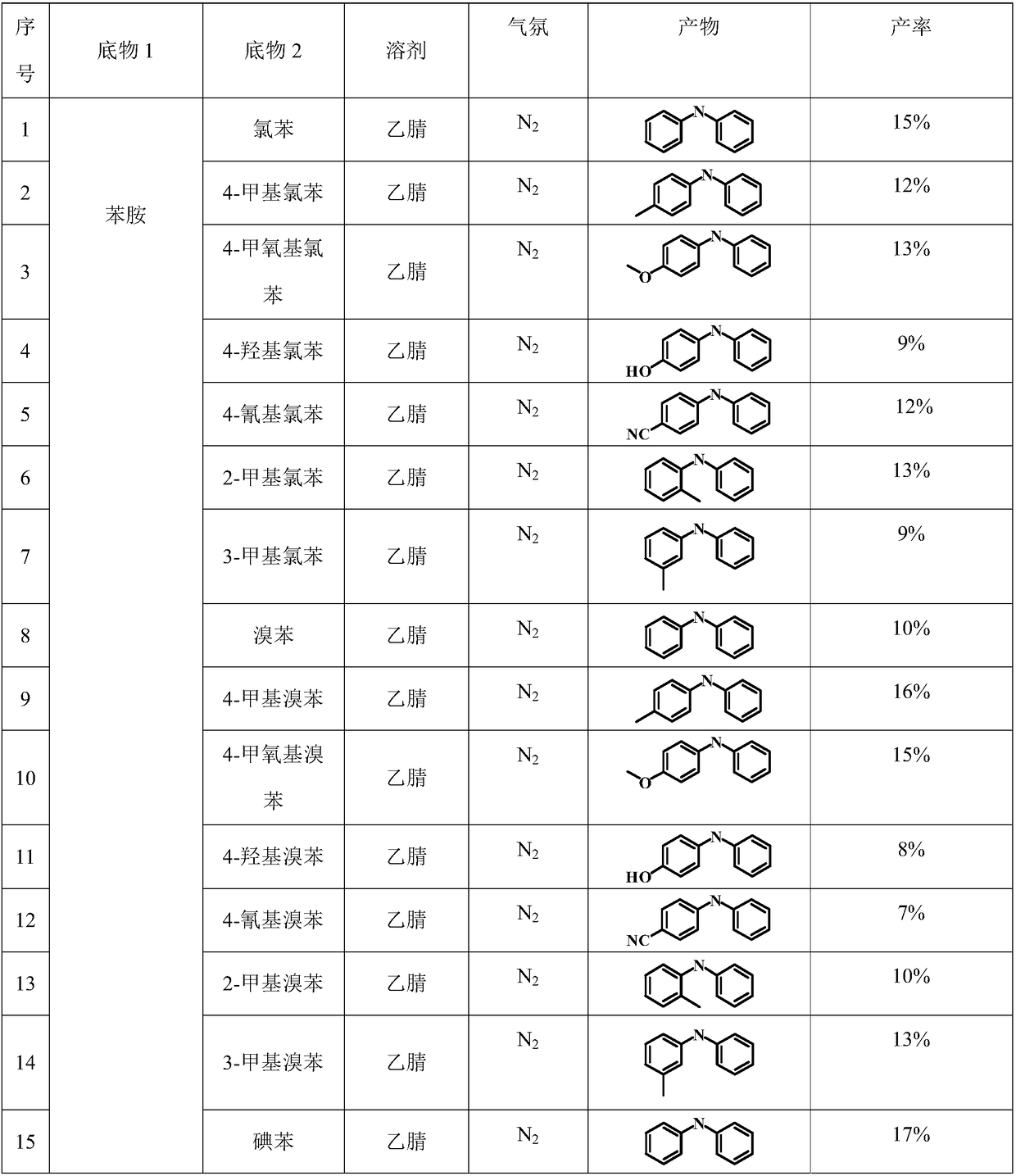
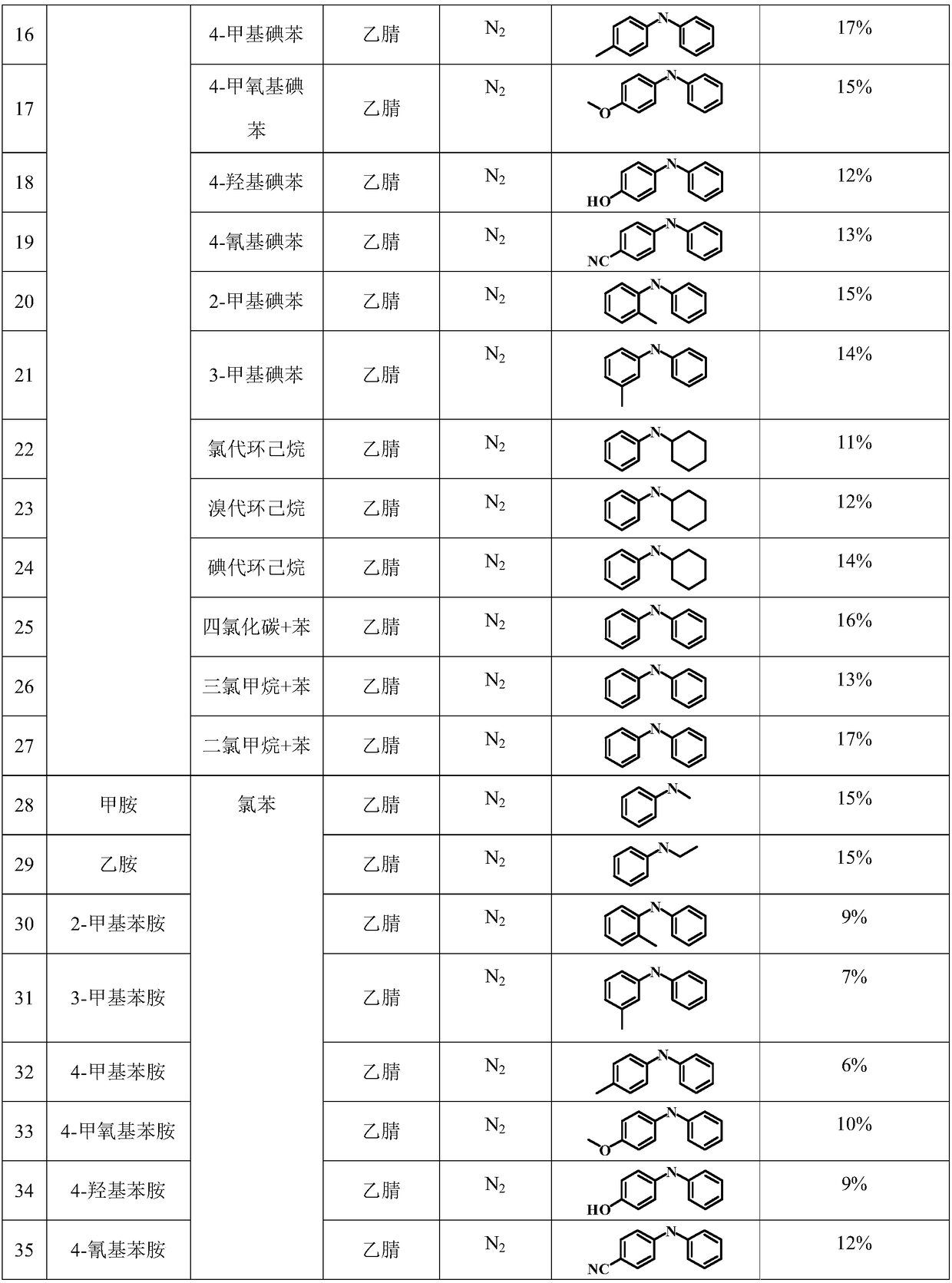
Examples
Embodiment 1
[0030] 1. Purification of solvent and reaction substrate
[0031] Measure 100 milliliters of acetonitrile, and carry out vacuum distillation in a vacuum distillation device to remove impurities and water contained in the solvent, and then transfer it to N 2 Keep in the protective glove box. The reaction substrates chlorobenzene and aniline were transferred to the glove box in the same way as above for subsequent use.
[0032] 2. N 2 Configure the reaction system under the atmosphere
[0033] Add 5 milliliters of solvent acetonitrile obtained in step (1) into a quartz reactor equipped with a magnetic stirrer, then add 100 microliters of chlorobenzene and 100 microliters of aniline to the container successively, then seal the reactor and move out of the glove box .
[0034] 3. Reaction system pretreatment
[0035] Place the sealed quartz reactor with the reaction substrate in an ultrasonic machine for 30 minutes, and then place it on a magnetic stirrer with a stirring rate ...
Embodiment 2
[0039] The specific organic amine synthesis method is basically the same as in Example 1 of this part, except that chlorobenzene is changed to 4-methylchlorobenzene.
Embodiment 3
[0041] The specific organic amine synthesis method is basically the same as in Example 1 of this part, except that chlorobenzene is changed to 4-methoxychlorobenzene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com