Improved method of differentiating from epidermal stem cell to pancreas cell
A technology of epidermal stem cells and pancreatic cells, applied in the field of non-embryonic pluripotent stem cells, can solve the problems of low market application value, unsuitable for large-scale promotion and use, and limited sources of embryonic stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of Epidermal Stem Cells
[0023] The foreskin skin of (human) healthy male after circumcision was taken to prepare epidermal cells and fibroblasts.
[0024] 1. Separation of epidermal cells: take 2cm long and 2mm wide skin, wash 3 times with PBS containing antibiotics; put in protease solution, digest at 4°C for 16 hours; take out the skin, peel off the epidermis and dermis; collect epidermal slices , placed in 0.25% trypsin / 0.02% EDTA (1:1) mixture, digested at 37°C for 15 minutes, terminated the digestion, filtered after a little pipetting, collected the cell suspension, discarded the supernatant by centrifugation, added fresh culture medium, and re- Suspend the cells, adjust the cell concentration to 1×10 3 / ml.
[0025] 2. Preparation of trophoblast cells: collect the above-mentioned dermis slices after peeling off the epidermis and dermis, place them in 625U / ml collagenase solution, collect the fibroblast suspension after digestion, and cult...
Embodiment 2
[0029] The preparation of embodiment 2 active stimulating peptides
[0030] Firstly, a random polypeptide library is constructed, wherein the random polypeptide described in the invention is a random amino acid sequence containing less than 30 (including 30). The polypeptide random library of the present invention is obtained by translation and transformation of the corresponding DNA library (DNA library used for screening CPP). The transformation method of polypeptide random library described in the present invention is a transformation method commonly used in the art. For example, chemical transformation method and electroporation transformation method, see "Molecular Cloning (Chinese Second Edition)" edited by J. Sambrook et al. Random polypeptides were mixed with epidermal stem cells. At this time, the basal medium was DMEM medium containing 10% fetal bovine serum and 100ng / ml activin A (Sigma). According to the difference in the differentiation index of epidermal stem ce...
Embodiment 3
[0031] Example 3 Differentiation Induction of Epidermal Stem Cells to Pancreatic Cells
[0032] Put the epidermal stem cells prepared in Example 1 into a culture dish covered with 1% Matrigel and induce with active stimulating peptides as follows:
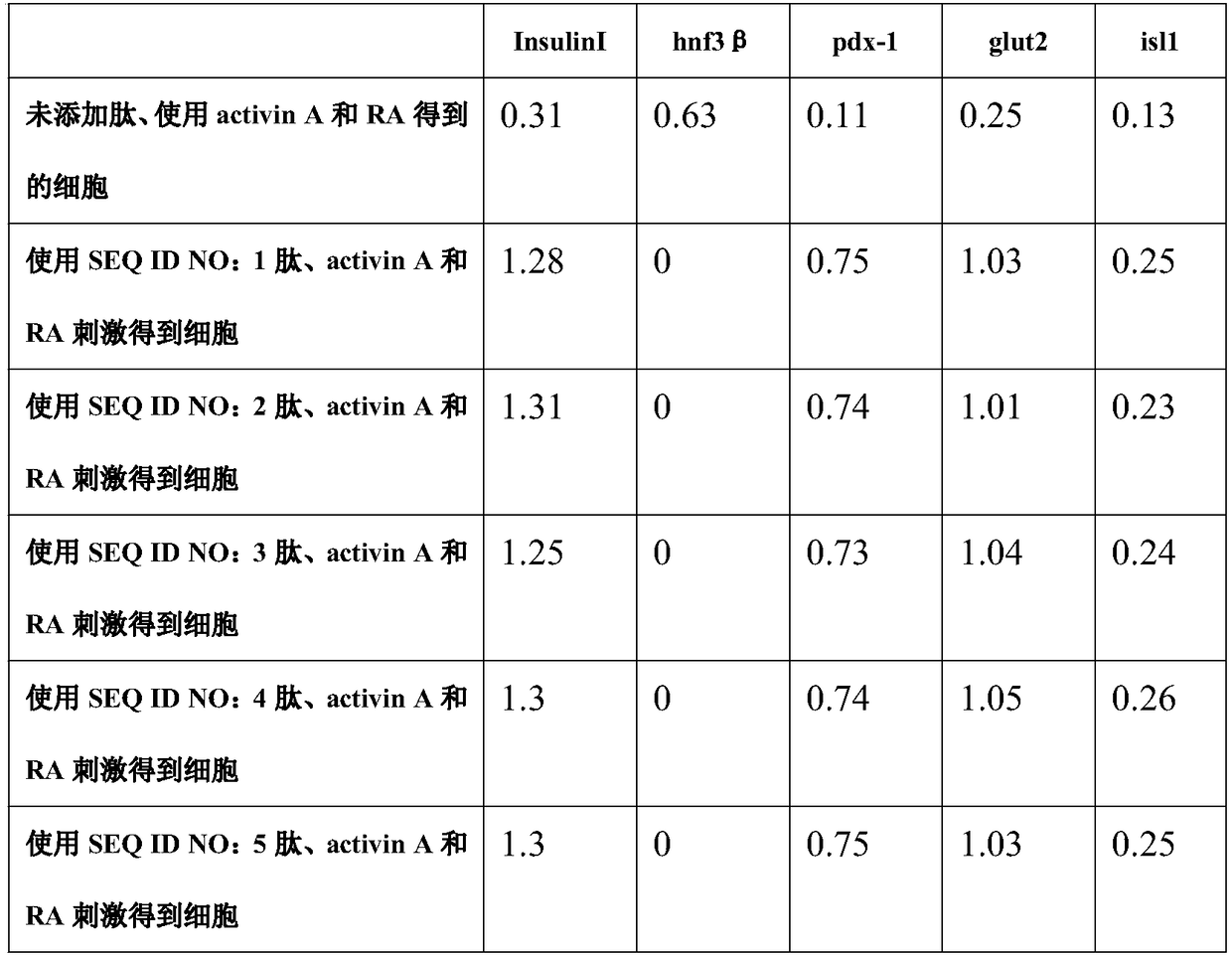
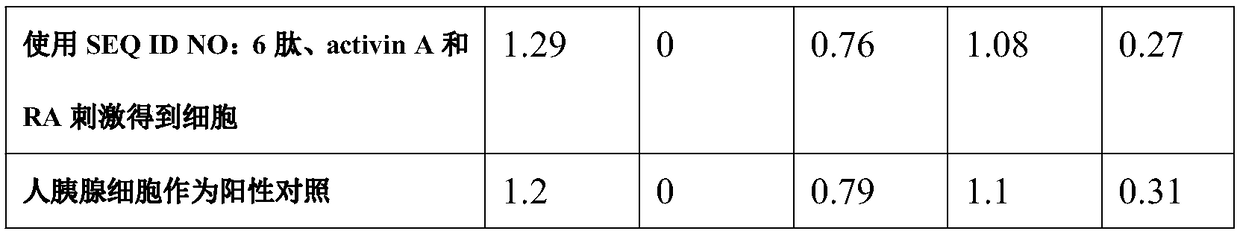
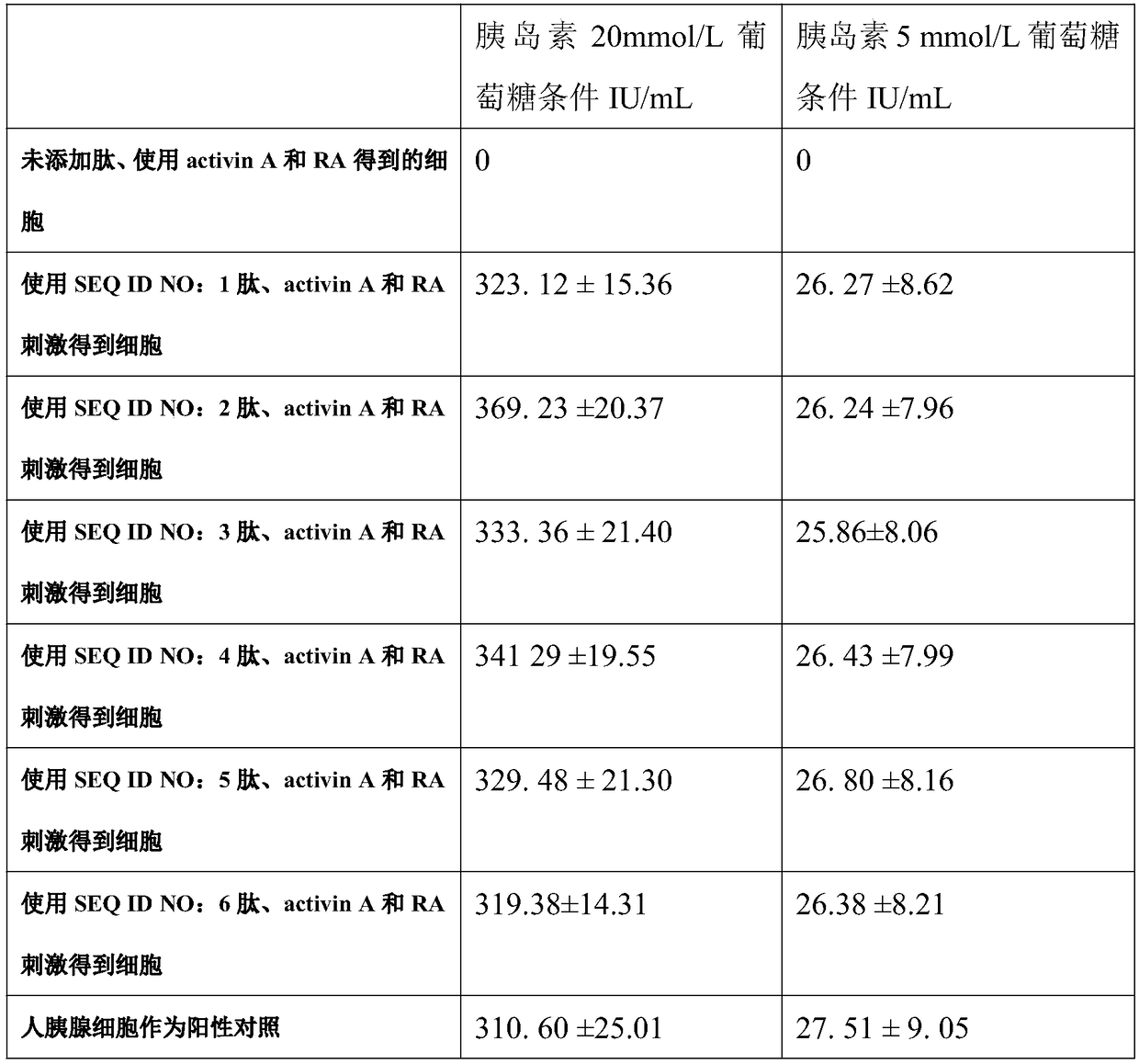
[0033] Two hours later, the epidermal stem cells began to spread. At this time, the medium was replaced with DMEM medium containing 10% fetal bovine serum and 100ng / ml activin A (Sigma) for 24 hours, and then the epidermal stem cells were changed to 10% fetal calf serum containing 10% fetal calf serum. Bovine serum and 10 mg / L active stimulating peptide (six different active peptides of SEQ ID NO: 1-6 were tested separately, and the active peptide was not added as a blank control) in DMEM for 6 hours to differentiate epidermal stem cells . Afterwards, differentiated epidermal stem cells were then treated with 10% fetal bovine serum and 1 x 10 -6 The DMEM medium of mol / L RA (Sigma) was further induced for 24 hours to obtain pancreat...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap