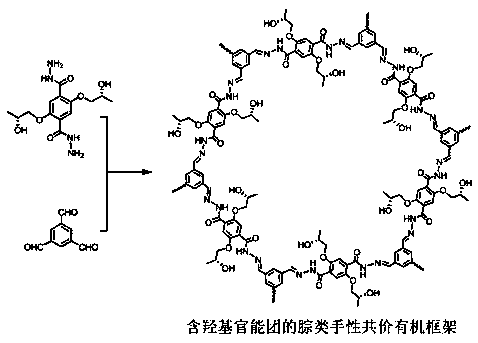
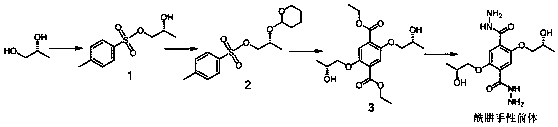
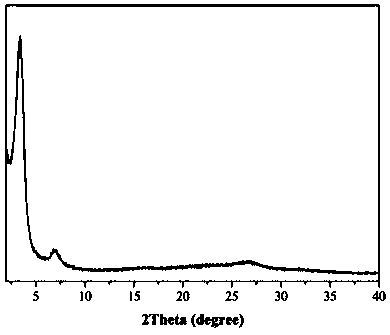
Hydrazone chiral covalent organic framework material containing hydroxyl as well as preparation method and application of hydrazone chiral covalent organic framework material
A covalent organic framework and chiral technology, applied in separation methods, chemical instruments and methods, and other chemical processes, can solve problems such as difficulty in maintaining crystallinity, low symmetry, and achirality, and achieve good crystallinity, The effect of high thermal stability and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Hydrazone chiral covalent organic framework materials containing hydroxyl functional groups provided by the present invention are composed of figure 1 The procedure shown was prepared. Specifically, with R Configuration hydrazide chiral precursor as an example, the R The chiral precursor of configuration hydrazide (10.3 mg, 0.03 mmol), tricarbaldehyde (3.2 mg, 0.02 mmol), 0.5 mL of dioxane and 0.5 mL of mesitylene were placed in a pressure-resistant reaction flask and mixed thoroughly After homogenization, 0.1 mL of acetic acid solution (6 M) was added. The resulting mixture was deoxygenated by argon replacement, quickly sealed, and placed in an oven preheated to 110° C. for crystallization reaction for 3 days. After the reaction was completed, it was cooled to room temperature, and the solid was collected by suction filtration, washed with 1,4-dioxane, tetrahydrofuran in sequence, and finally washed with acetone and dried in vacuum to obtain a chiral covalent organi...
Embodiment 2
[0064] Will R Configuration Hydrazide chiral precursor (20.5 mg, 0.06 mmol), trimesaldehyde (6.5 mg, 0.04 mmol), 0.5 mL of dioxane and 0.5 mL of mesitylene were placed in a pressure-resistant reaction flask and mixed thoroughly After homogenization, 0.1 mL of acetic acid solution (6 M) was added. The resulting mixture was deoxygenated by argon replacement, quickly sealed, and placed in an oven preheated to 110° C. for crystallization reaction for 3 days. Cool to room temperature after the reaction, collect the solid by suction filtration, rinse with 1,4-dioxane, tetrahydrofuran in sequence, and finally rinse with acetone and dry in vacuum to obtain a chiral covalent organic framework material.
Embodiment 3
[0066] Will R Conformational hydrazide chiral precursor (10.3 mg, 0.03 mmol), tricarbaldehyde (3.2 mg, 0.02 mmol), 0.5mL N , N’ - Dimethylformamide and 0.5 mL of mesitylene were placed in a pressure-resistant reaction flask, mixed well, and then 0.1 mL of acetic acid solution (3 M) was added. The resulting mixture was deoxygenated by argon replacement, quickly sealed, and placed in an oven preheated to 120° C. for crystallization reaction for 4 days. After the reaction, cool to room temperature, collect the solid by suction filtration, wash with tetrahydrofuran for several times, then wash with acetone once, and finally vacuum-dry to obtain the chiral covalent organic framework material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com