Preparation method of ambroxol hydrochloride impurity reference substance
A technology of ambroxol hydrochloride and impurity reference substances, which is applied in the field of preparation of ambroxol hydrochloride impurity reference substances, can solve the problems of increased risk, low repetition rate, and harsh reaction conditions, so as to reduce experimental risks and increase safety , The effect of stable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
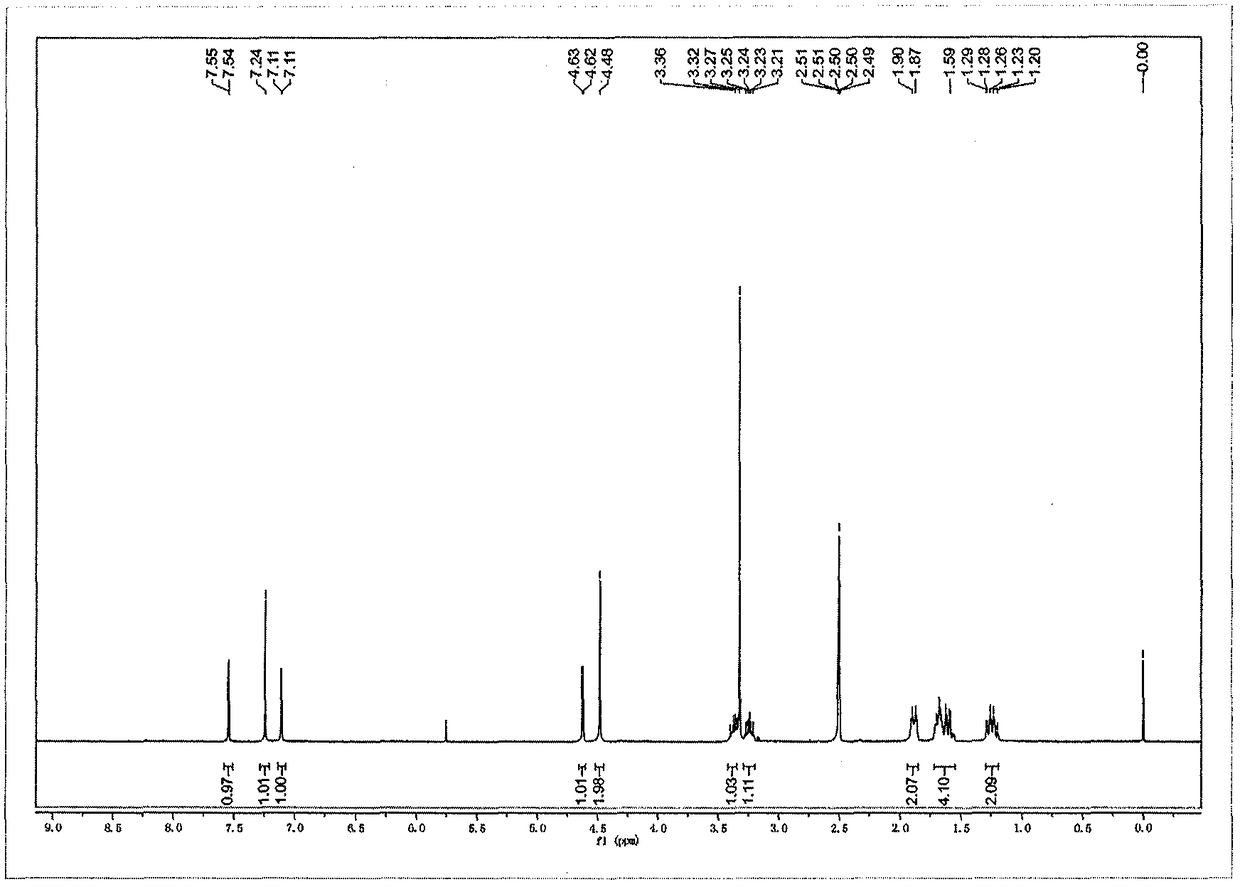
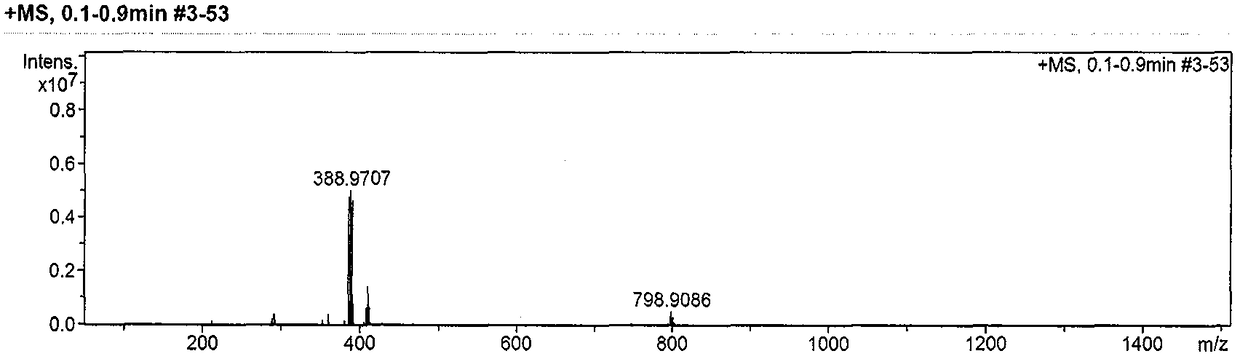
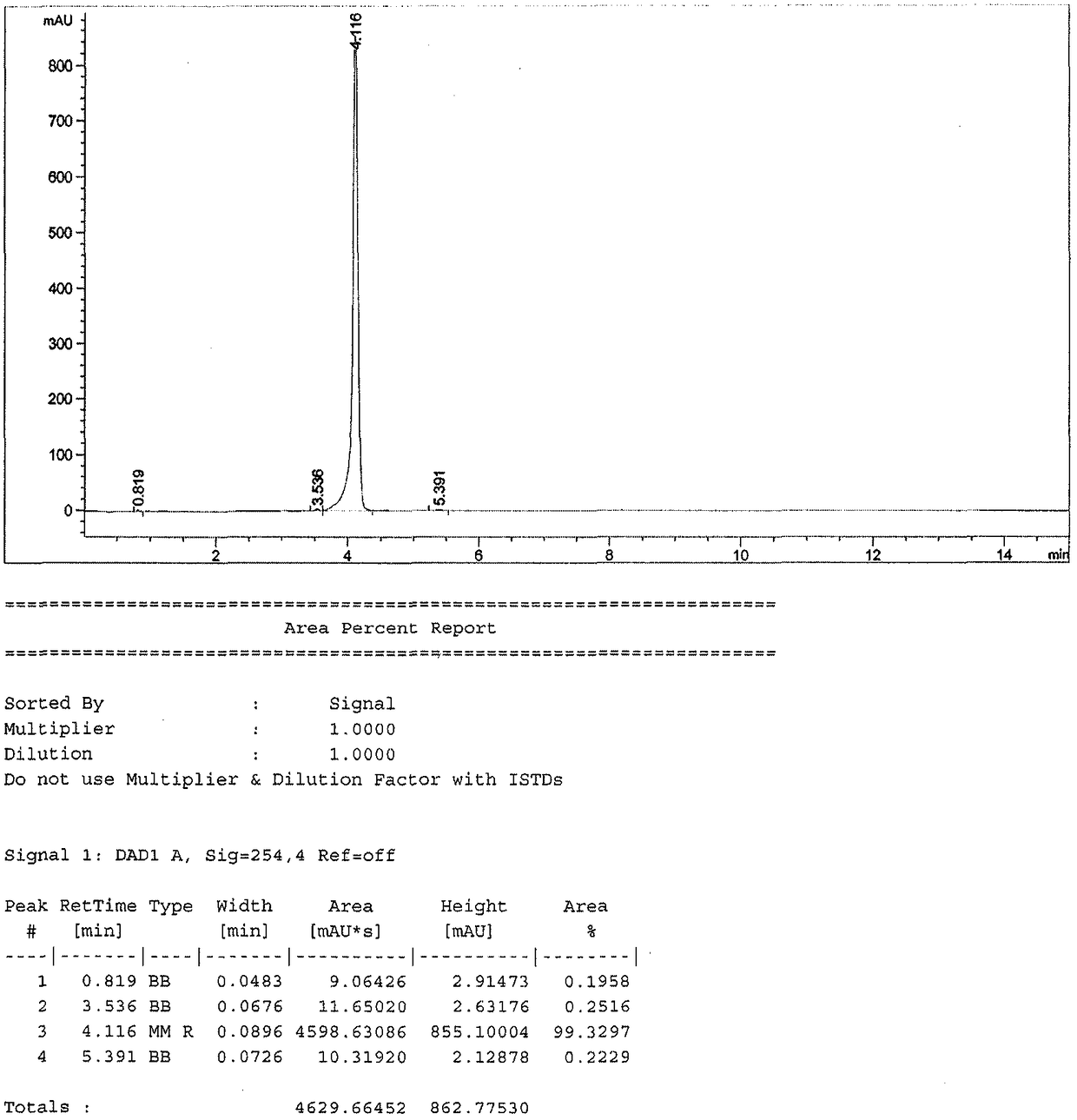
[0027] A kind of preparation method of ambroxol hydrochloride impurity reference substance, concrete steps are as follows: first, 1g ambroxol hydrochloride is dissolved in the dichloromethane solvent of 10mL, adds 0.6g saturated sodium bicarbonate solution and pH is adjusted to slightly alkaline , extracted with dichloromethane, the organic phase was concentrated and dried, and then the concentrated solution was dissolved by adding 10 mL of ethanol, then added 715 mg of triethyl orthoformate and reacted at 80°C for 8 hours, and the reaction was detected by TLC thin-layer chromatography. When hydrochloric acid After the ambroxol raw material disappeared completely, the reaction solution was cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a white solid, which was purified by silica gel column chromatography to obtain (1R, 4R)-4-(6,8-dibromo -3,4-dihydroquinazoline)-cyclohexanol pure product 900mg, yield 96.14%, purity 99.3%.
[0028...
Embodiment 2
[0031] A kind of preparation method of ambroxol hydrochloride impurity reference substance, concrete steps are as follows: first, 0.5g ambroxol hydrochloride is dissolved in the dichloromethane solvent of 8mL, adds 0.3g saturated sodium bicarbonate solution and pH is adjusted to weak base nature, extracted with dichloromethane, concentrated and dried the organic phase, then added 5mL of ethanol to the concentrated solution to dissolve, then added 256mg of trimethyl orthoformate and reacted at 60°C for 8 hours. The reaction was detected by TLC thin-layer chromatography. After the ambroxol raw material disappeared completely, the reaction solution was cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a white solid, which was purified by silica gel column chromatography to obtain (1R, 4R)-4-(6,8-dibromo -3,4-dihydroquinazoline)-cyclohexanol pure product 430mg, yield 97.86%, purity 99.0%.
Embodiment 3
[0033] A kind of preparation method of ambroxol hydrochloride impurity reference substance, concrete steps are as follows: first, 1g ambroxol hydrochloride is dissolved in the dichloromethane solvent of 5mL, adds 0.6g saturated sodium bicarbonate solution and pH is adjusted to slightly alkaline , extracted with dichloromethane, the organic phase was concentrated and dried, then the concentrated solution was dissolved by adding 5mL of ethanol, and then added 358mg of triethyl orthoformate and reacted at 70°C for 8 hours, and the reaction was detected by TLC thin-layer chromatography. After the bromoxol raw material completely disappeared, the reaction solution was cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a white solid, which was purified by silica gel column chromatography to obtain (1R,4R)-4-(6,8-dibromo- 849 mg of pure 3,4-dihydroquinazoline)-cyclohexanol, with a yield of 96.69% and a purity of 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com