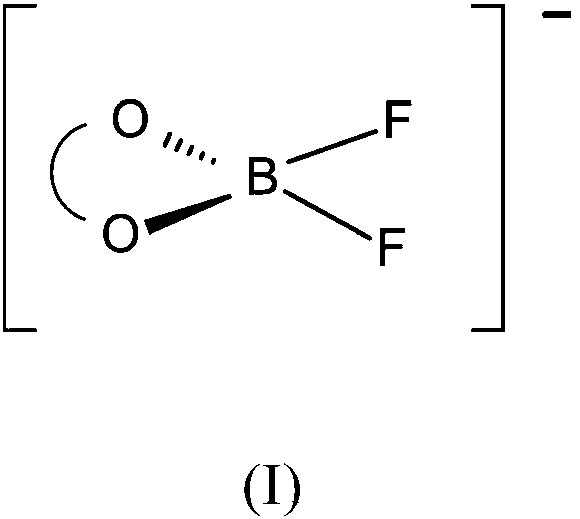
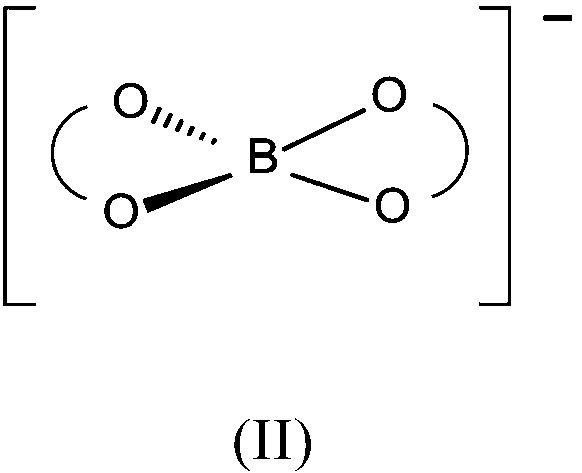
Preparation of difluoro chelato borate salts
A borate and chelating technology, applied in the field of preparation of difluoro chelate borate, can solve the problems of unfavorable fluorine, low efficiency loss and the like, and achieve the effects of easy acquisition, avoidance of purification steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] 38 g of oxalic acid dihydrate, 6 g of boric acid and 30 g of triethylamine were mixed with 150 mL of acetonitrile. 21 g of boron trifluoride-dihydrate were added and the reaction mixture was heated to 120-130° C. while distilling off volatile components. The residue was further dried at 120° C. and 10 mbar for 2 hours. After cooling, triethylammonium difluoro(oxalatoborate) was obtained as a colorless, solidified melt. Quantitative F-NMR showed a purity of 92%, the corresponding tetrafluoroborate (2%) and 6% bis(oxalatoborate) salt.
Embodiment 2
[0157] 38 g of oxalic acid dihydrate, 6 g of boric acid and 30 g of triethylamine were mixed with 150 mL of acetic acid. 21 g of boron trifluoride-dihydrate were added and the reaction mixture was heated to 120-130° C. while distilling off volatile components. The residue was further dried at 120° C. and 10 mbar for 2 hours. After cooling, triethylammonium difluoro(oxalatoborate) was obtained as a colorless, solidified melt. Quantitative F-NMR showed a purity of 93%, the corresponding tetrafluoroborate (3%) and 4% bis(oxalatoborate) salt.
Embodiment 3
[0159] 38 g of oxalic acid dihydrate, 6 g of boric acid and 30 g of triethylamine were mixed with 150 mL of methanol. 21 g of boron trifluoride-dihydrate were added and the reaction mixture was heated to 120-130° C. while distilling off volatile components. The residue was further dried at 120° C. and 10 mbar for 2 hours. After cooling, triethylammonium difluoro(oxalatoborate) was obtained as a colorless, solidified melt. Quantitative F-NMR showed a purity of 88%, the corresponding tetrafluoroborate (6%) and 6% bis(oxalatoborate) salt.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com