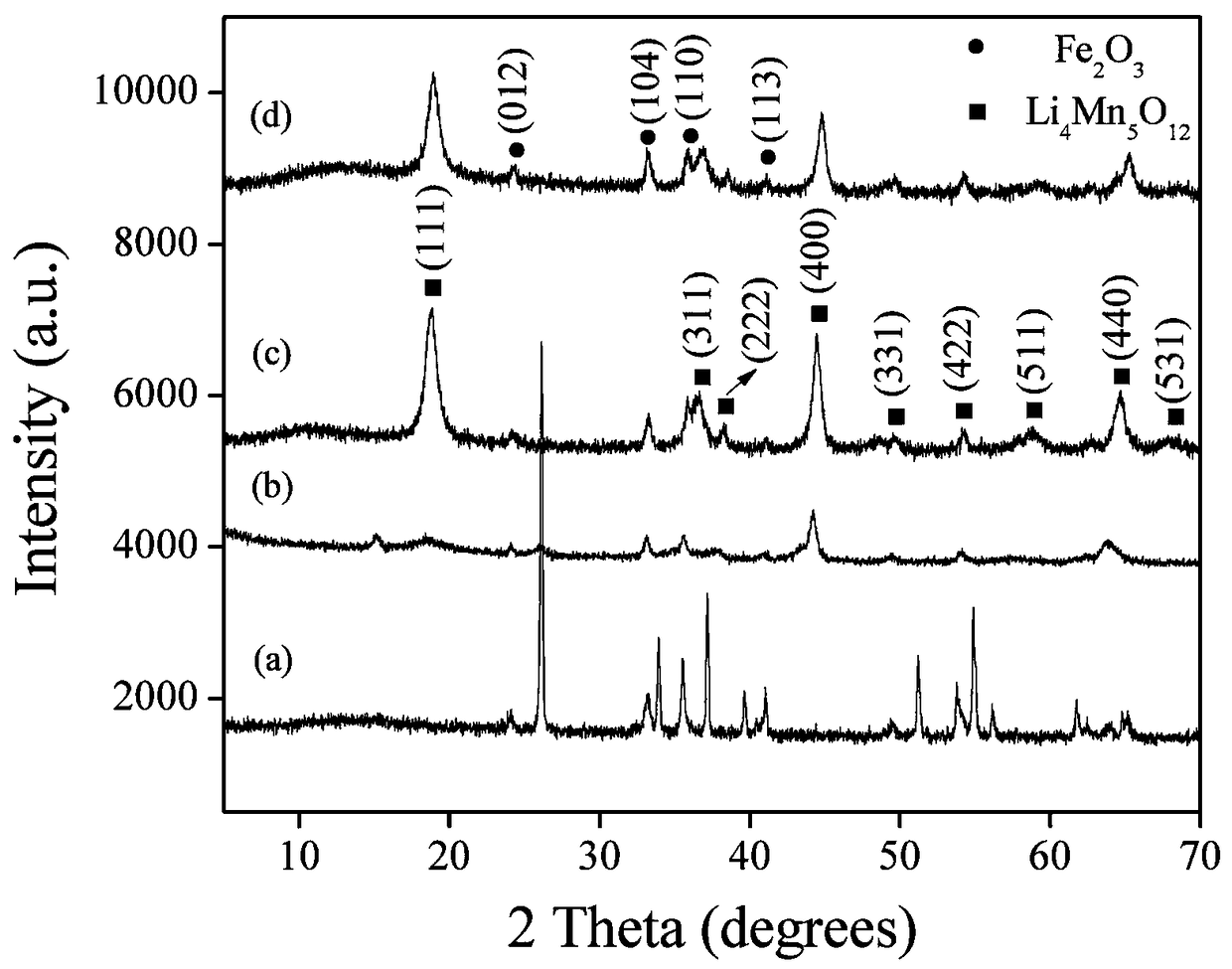
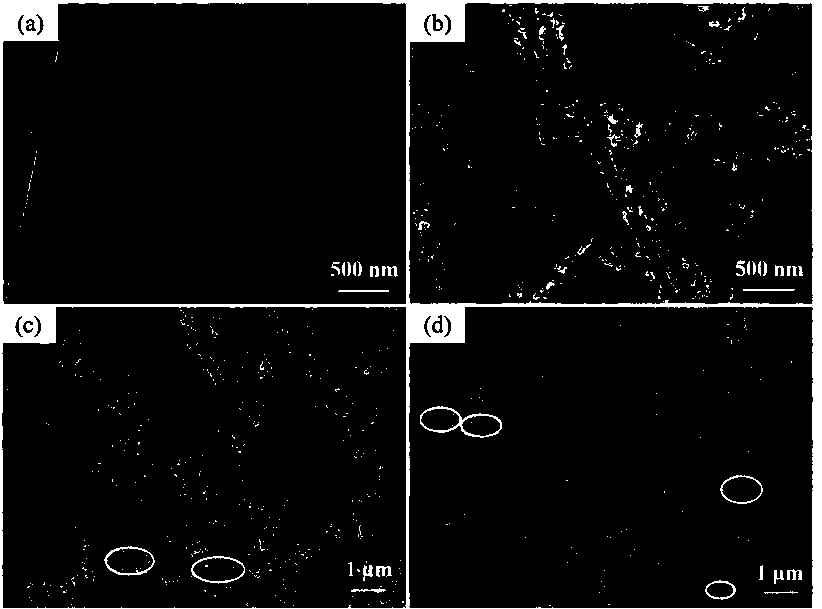
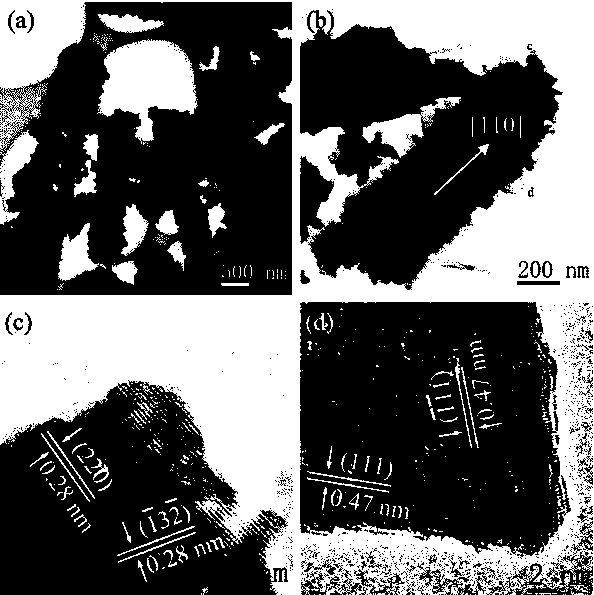
Preparation method of nanotube-shaped manganite lithium ion sieve absorbent
A manganese oxide and lithium ion technology is applied in the field of preparation of nano-tubular manganese oxide lithium ion sieve adsorbent, which can solve the problems of single adsorbent shape, low adsorption capacity, small specific surface area, etc., and achieve high-efficiency cycle Utilize performance, reduce production costs, and source a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method of a nanotubular manganese oxide lithium ion sieve adsorbent, comprising the steps of:
[0029] (1) Preparation of γ-MnOOH
[0030] Weigh 0.50 g KMnO respectively 4 and 0.334g FeSO 4Dissolve in a mixed solution of 65mL of secondary water and 5mL of ethanol, stir to mix evenly; pour the mixed solution into the lining of a 100mL reactor, and react at 140°C for 20 hours after the reactor is installed. After the reaction was finished, the reaction kettle was taken out, allowed to cool down to room temperature naturally, and the product was suction filtered, washed with water, and then washed with ethanol, and washed twice repeatedly. Put the solid product in a drying oven with a temperature of 55°C to dry the moisture;
[0031] (2) Synthesis of LiMnO 2
[0032] Weigh 0.62g of γ-MnOOH prepared in step (1) and slowly add it to 70mL of LiOH solution with a concentration of 2mol / L. After mixing evenly, place the solution in the lining of a 100mL reacto...
Embodiment 2
[0039] (1) Preparation of γ-MnOOH
[0040] Weigh 1.1g KMnO respectively 4 and 0.684g (NH 4 ) 2 Fe(SO 4 ) 2 Dissolve in a mixed solution of 65mL of secondary water and 5mL of ethanol, stir to mix evenly; pour the mixed solution into the lining of a 100mL reactor, and react at 158°C for 26 hours after the reactor is installed. After the completion, take out the reaction kettle, allow it to cool down to room temperature naturally, then filter the product with suction, wash with water, then wash with ethanol, and wash repeatedly for 3 times, put the solid product in a drying oven with a temperature of 60°C, and dry the water;
[0041] (2) Synthesis of LiMnO 2
[0042] Weigh 0.94g of γ-MnOOH prepared in step (1) and slowly add to 70mL of LiOH·H with a concentration of 2mol / L 2 O solution, after mixing evenly, place the solution in a 100mL reaction kettle lining, and react at a temperature of 120°C for 14 hours after the reaction kettle is installed. Suction filtration, wash...
Embodiment 3
[0049] (1) Preparation of γ-MnOOH
[0050] Weigh 1.5g KMnO respectively 4 and 1.024g NH 4 FePO 4 Dissolve in a mixed solution of 65mL of secondary water and 5mL of ethanol, stir to mix evenly; pour the mixed solution into the lining of a 100mL reactor, and react at 180°C for 30 hours after the reactor is installed. After the completion, the reaction kettle was taken out, allowed to cool naturally to room temperature, and the product was subjected to suction filtration, washed with water, and then washed with ethanol, and washed repeatedly for 4 times, and the solid product was placed in a drying oven at a temperature of 75°C to dry the water;
[0051] (2) Synthesis of LiMnO 2
[0052] Weigh 1.35 g of γ-MnOOH prepared in step (1) and slowly add it to 70 mL of LiOH·H with a concentration of 2 mol / L 2 O solution, after mixing evenly, place the solution in a 100mL reactor lining, and react at a temperature of 130°C for 20 hours after the reactor is installed. After the react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com