Preparation method of 1H-1,2,3-triazole
A 1H-1, 1.1H-1 technology, applied in the direction of organic chemistry, can solve the problems of difficult purification, decarboxylation, large amount of wastewater, etc., to improve the safety of process operation, simple and convenient environmental protection treatment, and convenient recycling of solid waste Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
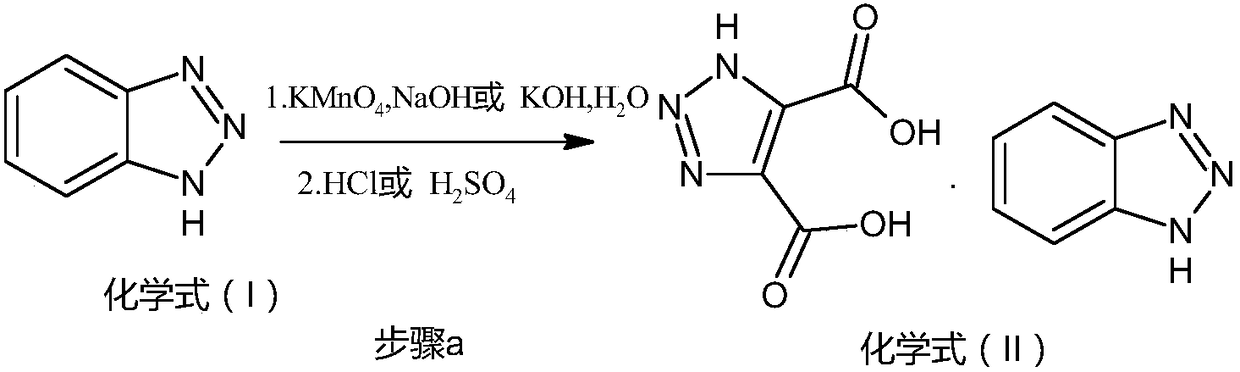
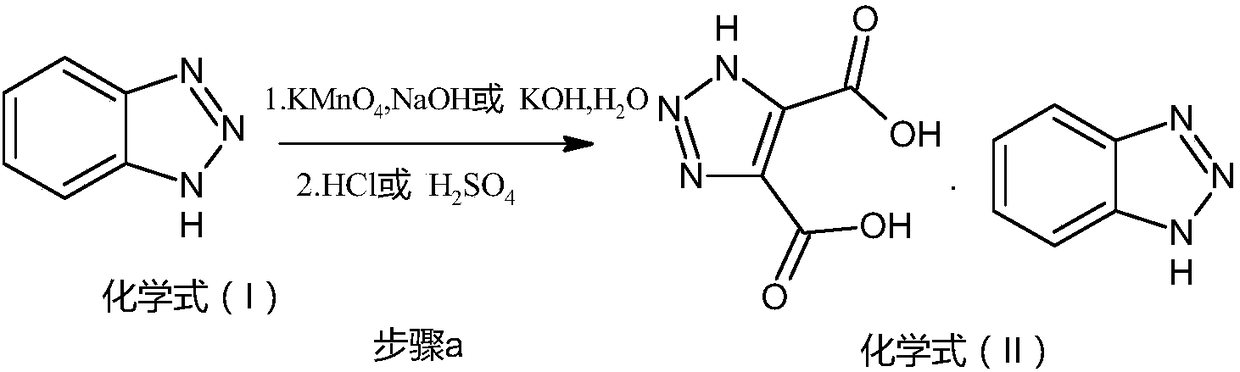
[0041] 1, the preparation of acid salt (chemical formula (II) compound)
[0042] Put 36g (0.3mol) of benzotriazole, 360ml of water, and 6g (0.15mol) of sodium hydroxide into a 1L reaction flask, raise the temperature to 40°C and add 94.8g (0.6mol) of potassium permanganate in batches to control the internal temperature 40°C, after the addition is complete, keep the reaction at 40°C for 6 hours. Heat preservation ratio, heat filter to remove manganese dioxide solid, rinse with hot water, drain, cool the filtrate to 25°C, add 5% hydrochloric acid dropwise to adjust pH=2, stir for 30min, then cool to 0°C and stir for 2 hours. Suction filtration, rinse with water, and drain. Dry in a hot-air circulating oven at 70°C for 8 hours to obtain 35.63 g of off-white solid chemical formula (II), with a yield of 86%.
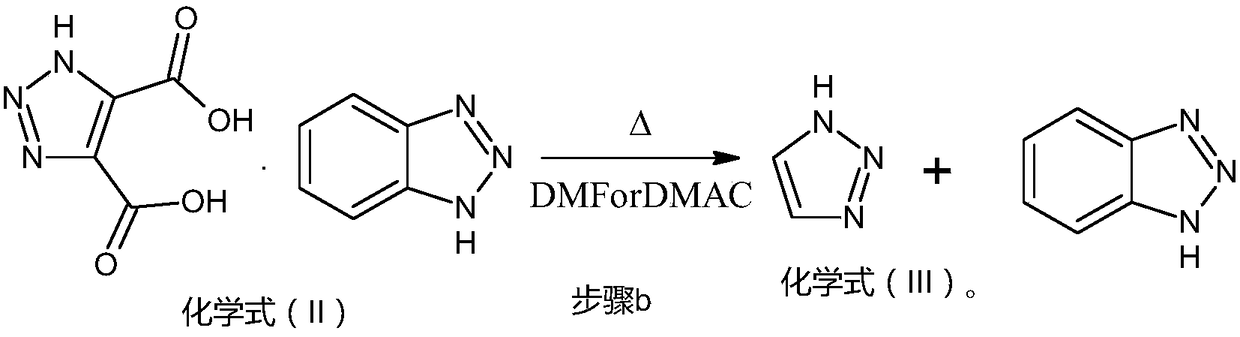
[0043] 2. Preparation of 1H-1,2,3-triazole (chemical formula (Ⅲ) compound)
[0044] Put 1000g of chemical formula (II) into a 3L flask, put in 2000ml of DMF, stir and rais...
Embodiment 2
[0046] 1, the preparation of acid salt (chemical formula (II) compound)
[0047]36g (0.3mol) of benzotriazole, 720ml of water, 25.2g (0.45mol) of potassium hydroxide were put into a 1L reaction flask, and 189.6g (1.2mol) of potassium permanganate was added in batches when the temperature was raised to 60°C. The temperature was 60°C, and the addition was completed, and the reaction was kept at 60°C for 3 hours. Heat preservation ratio, hot filtration to remove manganese dioxide solids, rinse with hot water, drain, cool the filtrate to 20-25°C, add concentrated sulfuric acid dropwise to adjust the pH of the solution to 2, stir for 30min, then cool to 0-5°C and stir 1 to 2 hours. Suction filtration, rinse with water, and drain. After vacuum drying at 80°C for 7 hours, 38 g of off-white solid chemical formula (II) was obtained, with a yield of 91%.
[0048] 2. Preparation of 1H-1,2,3-triazole (chemical formula (Ⅲ) compound)
[0049] Put 200g of chemical formula (II) into a 3L ...
Embodiment 3
[0051] 1, the preparation of acid salt (chemical formula (II) compound)
[0052] Put 36g (0.3mol) of benzotriazole, 1440ml of water, and 24g (0.6mol) of sodium hydroxide into a 2L reaction flask, raise the temperature to 80°C and add 284.4g (1.8mol) of potassium permanganate in batches to control the internal temperature 80°C, after the addition is complete, keep the reaction at 100°C for 1 hour. Heat preservation ratio, hot filtration to remove manganese dioxide solids, rinse with hot water, drain, cool the filtrate to 20-25°C, add 5% sulfuric acid dropwise to adjust the pH of the solution to 2, stir for 30min, then cool to 0°C and stir for 2 Hour. Suction filtration, rinse with water, and drain. After vacuum drying at 100°C for 6 hours, 38 g of off-white solid chemical formula (II) was obtained, with a yield of 91%.
[0053] 2. Preparation of 1H-1,2,3-triazole (chemical formula (Ⅲ) compound)
[0054] Put 200g of chemical formula (II) into a 3L flask, put in 2000ml of DMA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com