Novel application of herba cistanche phenylethanoid glycoside in improving sleep and application thereof
A phenethyloside and a technology for improving sleep are applied to the new use of Cistanche deserticola phenethyloside for improving sleep and its application field, which can solve the problems of no reports on improving sleep function, and achieve the effect of high yield and sleep improvement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation and content determination of phenylethanol glycosides in Cistanche deserticola.
[0025] 1. Extraction process: desert cistanche decoction pieces (batch number: C170160, Dalian Quanjian Chinese Medicine Decoction Pieces Co., Ltd.), crushed into 20-40 mesh coarse powder, weighed 1 kg, refluxed with 12 L 70% ethanol (volume ratio), 80 °C Extract 2 times, 2 hours each time; combine the extracts, concentrate under reduced pressure at 50°C to a paste, add a small amount of water to make a suspension of 1 L, and obtain the Cistanche deserticola extract solution.
[0026] 2. Purification process: Take the extract of Cistanche deserticola and put it on a D101 macroporous adsorption resin column. The volume ratio of resin to crude drug is 1:1, and the diameter-to-height ratio of the macroporous adsorption resin column is 1:9. Impurities were then eluted with 4 BV of 60% ethanol, the eluate was concentrated under reduced pressure at 50°C, and vacuum-dried at...
Embodiment 2
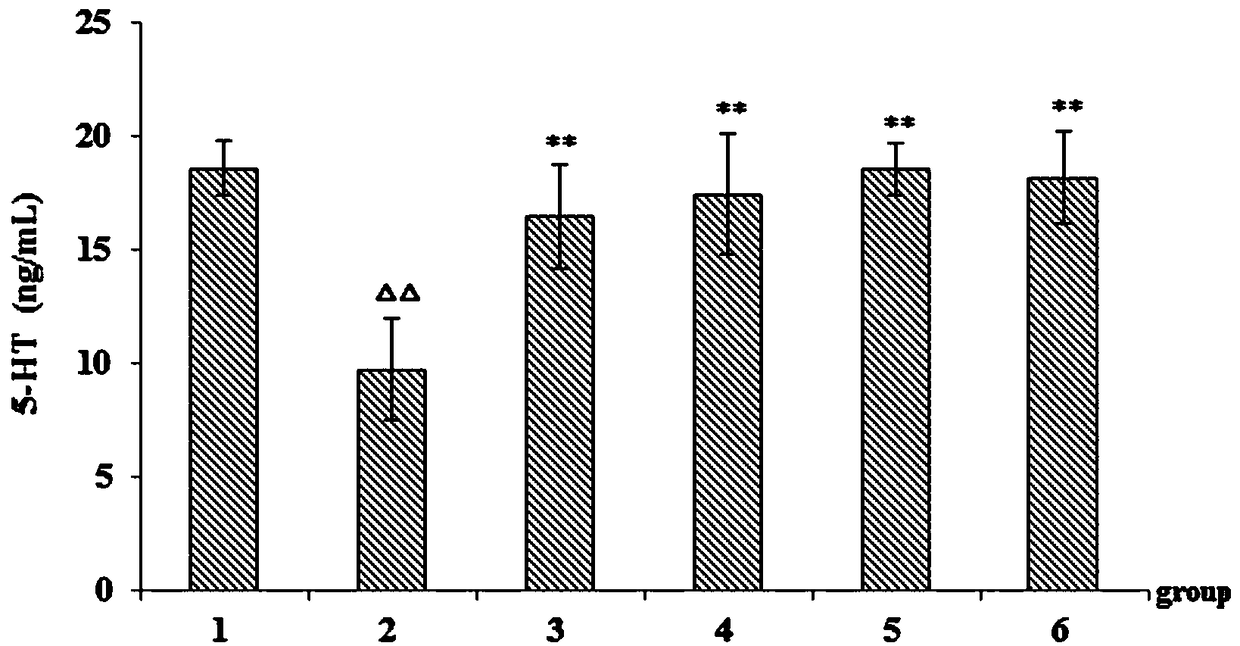
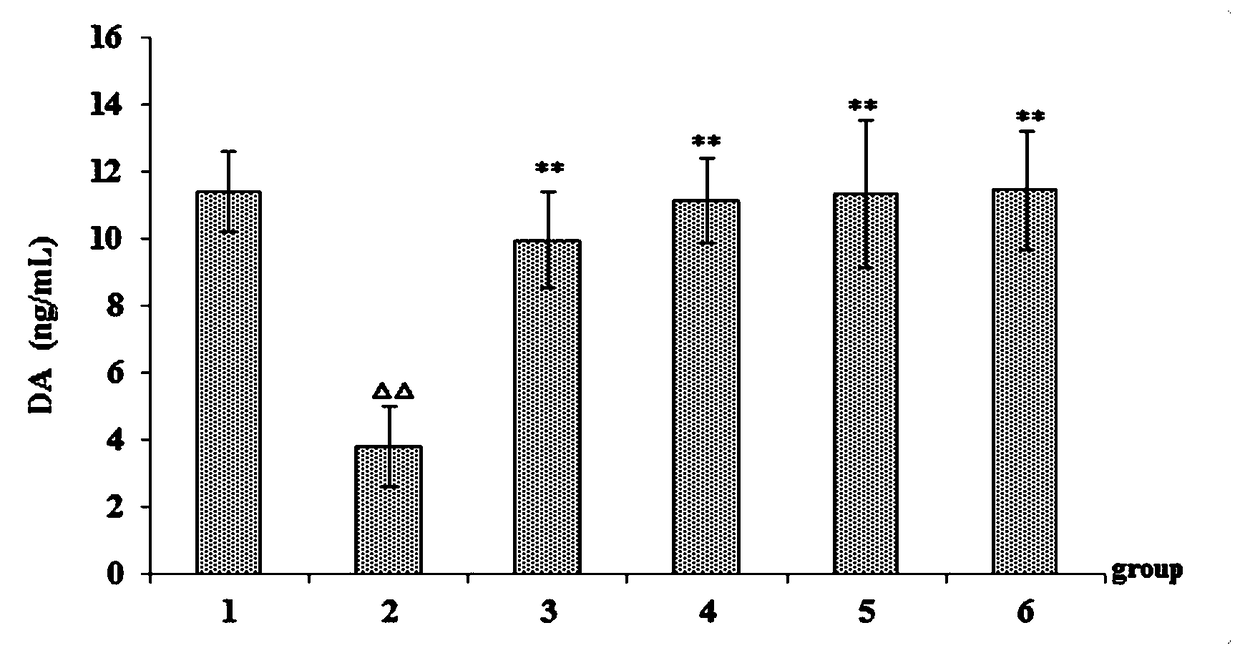
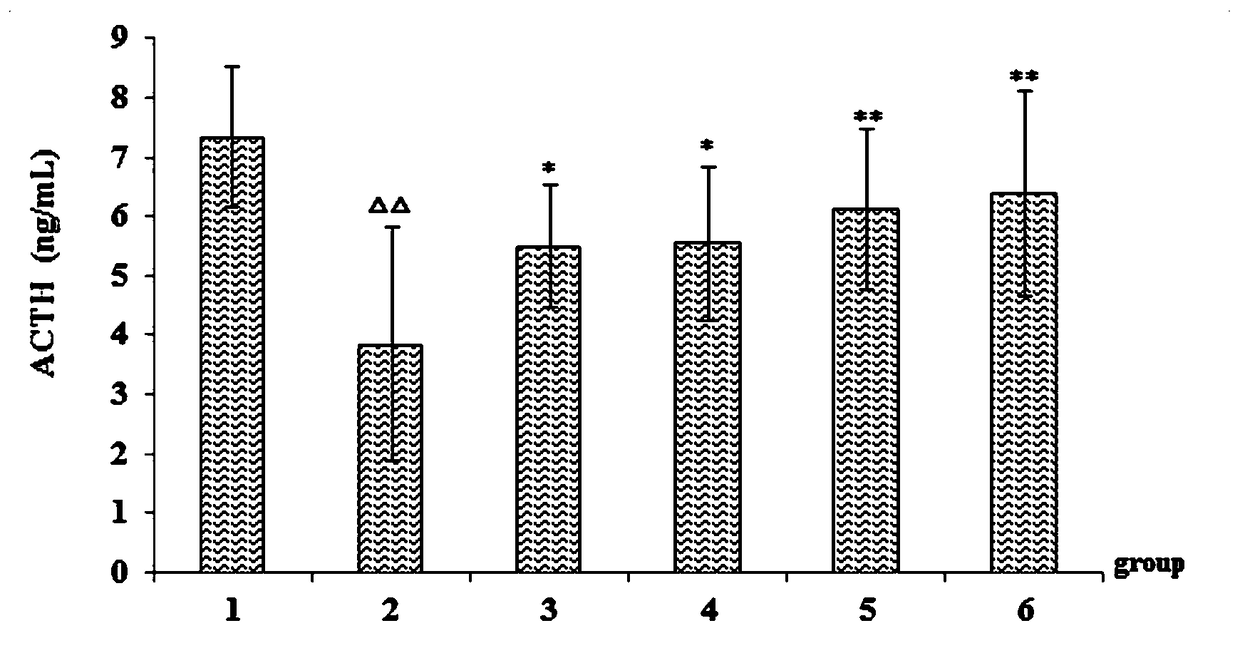
[0034] Example 2 Study on improvement of sleep function of asthenic insomnia model mice by phenylethanol glycoside of Cistanche deserticola.
[0035] 1. Experimental materials.
[0036] 1.1 Animals.
[0037] 60 ICR mice, male, weighing 20-22 g, 6-8 weeks old, provided by Liaoning Changsheng Biotechnology Co., Ltd. ) ℃, relative humidity 65%, and normal circadian rhythm to adjust the light time environment, eat standard feed and drink water freely.
[0038] 1.2 Main reagents and equipment.
[0039] Normal saline (batch number: 1607211208, Jilin Dubang Pharmaceutical Co., Ltd.); p-chlorophenylalanine (Sigma, USA); 5-HT, DA, ACTH kit (batch number: 201807, Shanghai Youyou Biotechnology Co., Ltd. ); diazepam tablets (batch number: 20171005, Jining Ankang Pharmaceutical Co., Ltd.); Cistanche cistanche phenylethanol glycoside extract (prepared by Example 1); TDZ4-WS low-speed desktop centrifuge (Changsha Xiangyi Centrifuge Instrument Co., Ltd. ); HH-S type electric heating const...
Embodiment 3
[0055] Example 3 Using the test method for improving sleep function to evaluate the research on improving sleep function of phenylethanol glycosides from Cistanche deserticola.
[0056] 1. Experimental materials.
[0057] 1.1 Animals.
[0058] 120 ICR mice, male, weighing 20-22 g, 6-8 weeks old, provided by Liaoning Changsheng Biotechnology Co., Ltd. ) ℃, relative humidity 65%, and normal circadian rhythm to adjust the light time environment, eat standard feed and drink water freely.
[0059] 1.2 Main reagents.
[0060] Cistanche cistanche phenylethanol glycoside extract (prepared in Example 1), pentobarbital sodium (US Sigma Company), barbital sodium (US Sigma Company).
[0061] 1.3 Grouping and dose selection.
[0062] 120 mice were divided into 3 batches for the experiment. The first batch of animals was used for direct sleep experiment and prolonged pentobarbital sodium sleep time experiment, and the second batch of animals was used for pentobarbital sodium subthreshol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com