Butynediol semi-hydrogenation bimetallic catalyst, preparation method and applications thereof
A bimetallic catalyst and butynediol technology, applied in the field of catalysis, can solve the problems of high hydrogen pressure, poor catalyst stability, aggravated production costs, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
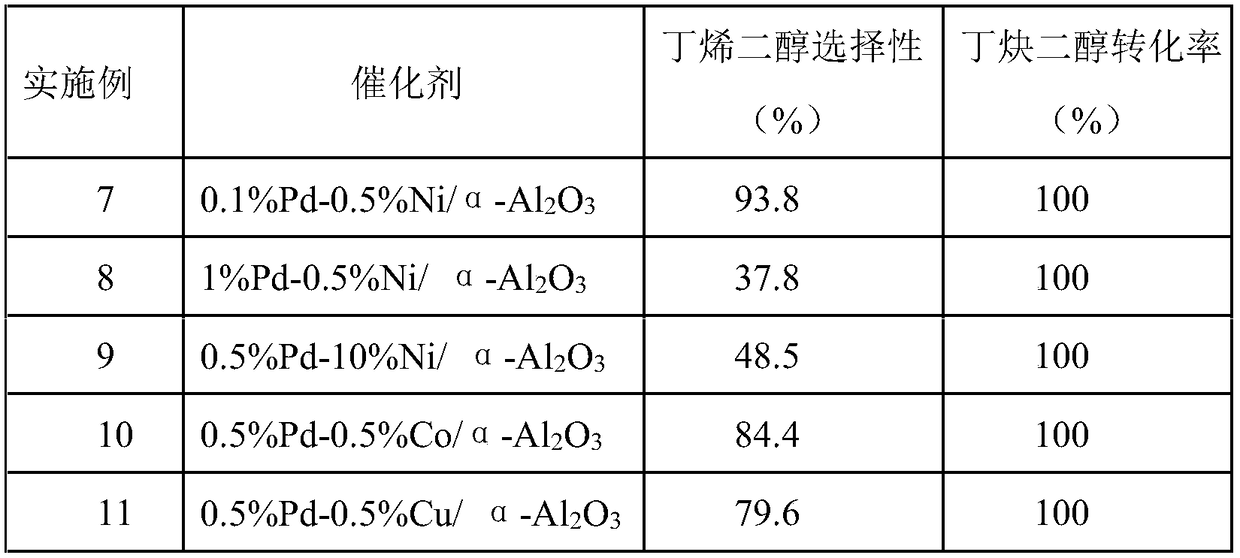
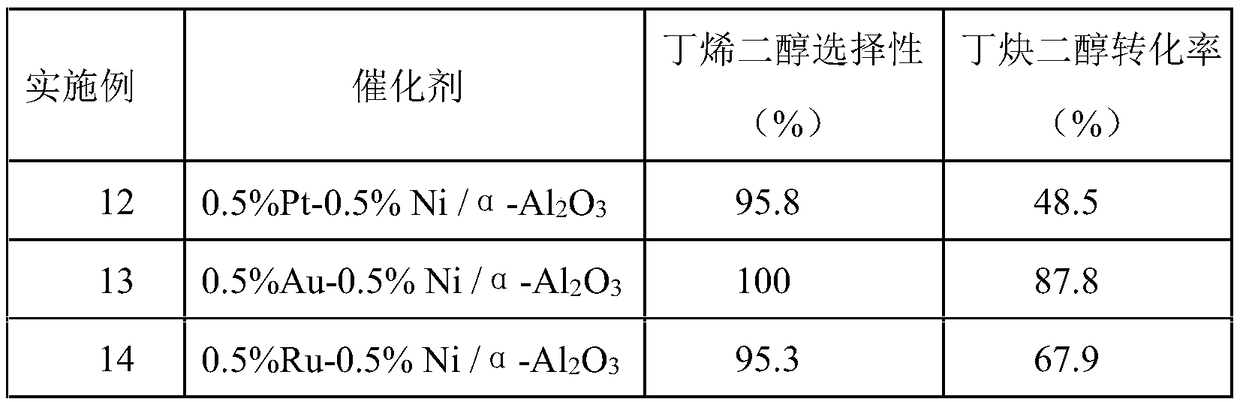
Embodiment 1
[0027] Catalyst preparation:
[0028] (1) 0.0505g NiCl 2 ·6H 2 O and 0.3g PVP-K30 were added to the Erlenmeyer flask, and a mixed solvent composed of 82.5mL ultrapure water and 12.5mL ethanol was added to dissolve, stirred for 10min, and NaOH solution was added to adjust the pH value of the mixed solution to between 9-14;
[0029] (2) Add 25.0mL 85wt% hydrazine hydrate and stir for 10min;
[0030] (3) Add 2.5gα-Al 2 o 3 And continue to stir for 10min;
[0031] (4) Transfer the mixture in the Erlenmeyer flask to a hydrothermal synthesis kettle (sealed with tape), stir magnetically at room temperature for 18 hours and then filter, wash the obtained solid with ethanol and ultrapure water, dry it in vacuum at 60°C for 6 hours, and crush it Get solid powder;
[0032] (5) Pipette PdCl 2 Solution 10.6mL (concentration: 0.00118gPd / mL water) in a 250mL Erlenmeyer flask, the solid powder 2.545g prepared in step (4) was added to 10.6mL (concentration: 1.18mgPd / mL water) PdCl 2 Ul...
Embodiment 2
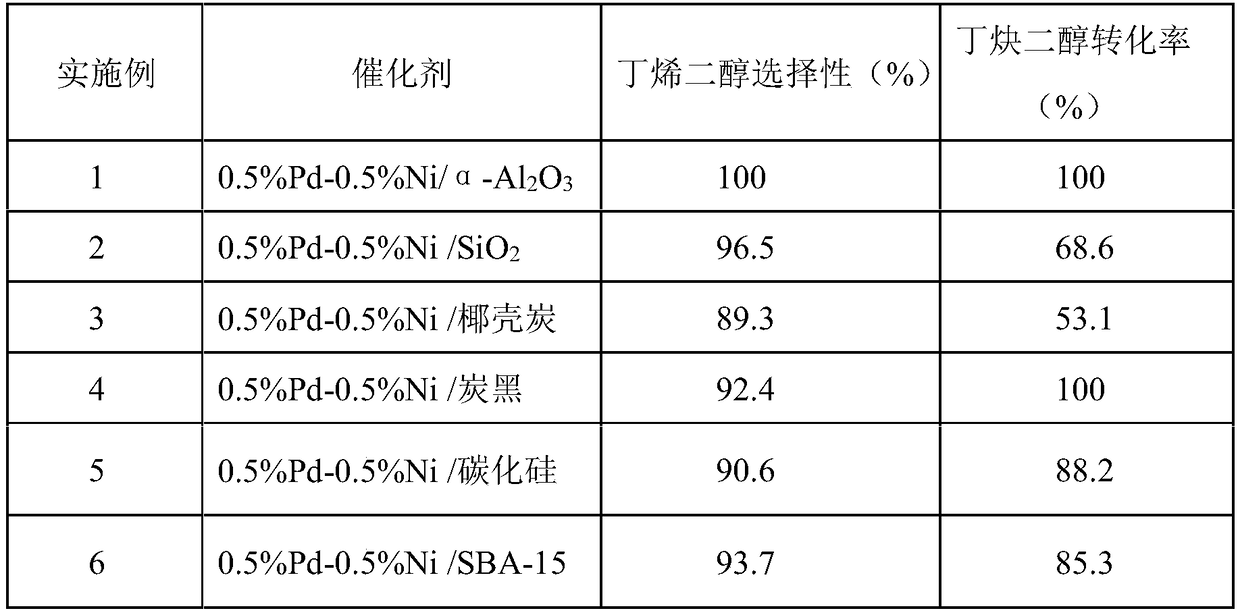
[0037] Take 2.5g SiO 2 Carrier instead of α-Al 2 o 3Carrier, the calcination temperature is adjusted to 500°C, the calcination time is adjusted to 3h, and the rest of the conditions are the same as in Example 1 to prepare the catalyst 0.5%Pd-0.5%Ni / SiO 2 . The evaluation conditions are the same as
[0038] Embodiment 1, the evaluation results are shown in Table 1.
Embodiment 3
[0040] Take 2.5g coconut shell charcoal carrier to replace α-Al 2 o 3 Carrier, all the other steps can make catalyst 0.5%Pd-0.5%Ni / coconut shell charcoal with embodiment 1. Evaluation conditions are the same as in Example 1, and the evaluation results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com