Preparation method of high dispersity Pt-Cu alloy nano-particles
A technology of alloy nanoparticles and high dispersibility, applied in nanotechnology, electrodes, electrolysis process, etc., can solve the problems of high cost of oleylamine and tetradecanediol, inability to obtain the target product in one step, and affecting the application performance of materials. , to achieve the effect of easy operation, easy industrial production and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation 1 of Pt-Cu alloy
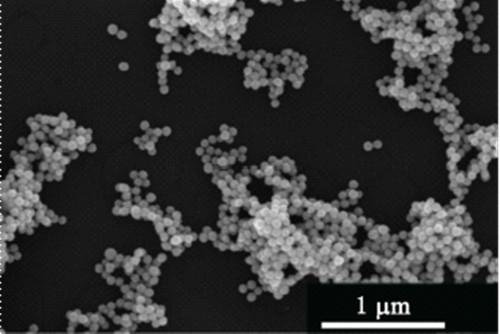
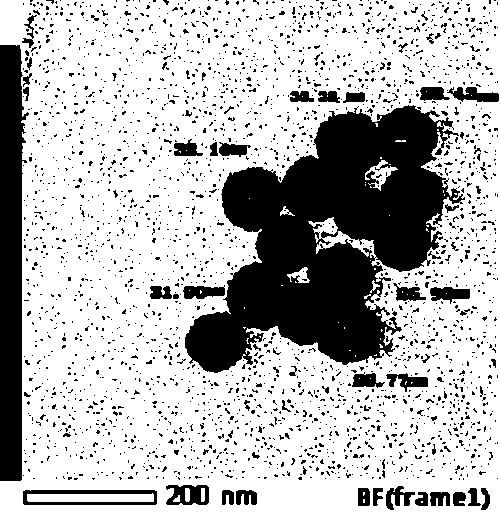
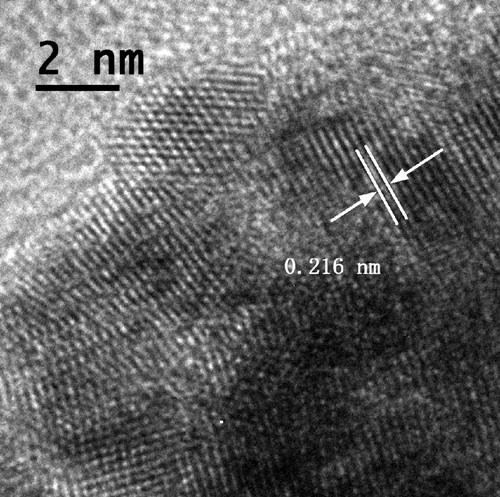
[0040] With deionized water as the solvent and copper chloride as the solute, configure 7.5 ml of copper chloride solution with a concentration of 15 mmol / L; add 0.5 g of cetyltrimethylammonium chloride, and place in a constant temperature water bath at 95 degrees Celsius to evenly Stir; inject ascorbic acid solution with a volume of 2.5 ml and a concentration of 1.0 mol / L; when the solution turns from blue to transparent, add chloroplatinic acid with a volume of 1 ml and a concentration of 10 mmol / L, stir and heat for 20 minutes. The obtained product is separated by a centrifuge, dispersed in deionized water for ultrasonication, secondly centrifuged, dispersed in ethanol, and placed in a blast drying oven at 50 degrees after being centrifuged again for drying.
[0041] We first performed an X-ray diffraction test on the sample. from figure 1 It can be seen that the diffraction peaks of the obtained product are located betw...
Embodiment 2
[0045] Example 2: Verification of the effect of cetyltrimethylammonium chloride.
[0046] In order to verify the effect of adding cetyltrimethylammonium chloride in the whole preparation, we implement the following experiments: the operation in Example 1 "adding 0.5 gram of cetyltrimethylammonium chloride" is removed, and other conditions constant.
[0047] pass Figure 7 The X-ray diffraction pattern characterization we can obtain, the product of embodiment 2 is Cu 3 Pt and Cu 2 O complex. Field emission scanning electron microscopy results can be seen: the complex is granular, with a particle size of 0.5 to 1.2 microns (see Figure 8 ). This example proves that without the participation of cetyltrimethylammonium chloride, the reduction of divalent Cu ions is too fast, and some of the divalent Cu ions remain in Cu without being reduced to elemental Cu. 2 In the O stage, alloys with higher purity cannot be produced. Example 2 proves to us that the addition of cetyltrime...
Embodiment 3
[0048] Embodiment 3: the effect verification of chloroplatinic acid
[0049] In order to verify the effect of chloroplatinic acid in the preparation of Pt-Cu alloys, we implement the following experiments: in Example 1, "the solution is to be changed from blue to transparent, add 1 milliliter, and the concentration is 10 mmol / liter of platinum chloride Acid" step is deleted, and other operations remain unchanged. The results showed that no precipitated product was produced after 20 minutes or even longer reaction time. This example proves that ascorbic acid cannot reduce the divalent Cu source to elemental Cu with the participation of chlorine-free platinum acid. Example 3 proves to us that chloroplatinic acid plays the role of providing Pt source and promoting the conversion of divalent Cu to zero-valent Cu in the reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap