Tyrosine-derived amygdalin-loaded hydrogel preparation method and application
A technology of amygdalin and hydrogel, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve traumatic brain injuries that cannot be directly affected and are difficult to achieve curative effect and other problems, to achieve good anti-inflammatory effect, low cost and easy injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Weigh 5.0 mg / mL of Fmoc-Tyr-OH solid powder, dissolve it in 100 μL of dimethyl sulfoxide, and ultrasonically disperse it for 30 seconds to completely dissolve it to prepare a Fmoc-Tyr-OH stock solution with a concentration of 50 mg / mL; weigh Take 5.0 mg of amygdalin in a centrifuge tube, add 1.0 mL of ultrapure water to dissolve it, and prepare a stock solution of amygdalin with a concentration of 5 mg / mL.
[0038] Take 50μL of Fmoc-Tyr-OH stock solution and add it to a washed and dried 5.0mL screw bottle, add 970μL of amygdalin stock solution, shake it up quickly, and let it stand for 3 minutes to form a clear and transparent tyrosine derivative Hydrogel loaded with amygdalin.
[0039] SEM experimental steps:
[0040]Silicon wafers were ultrasonically cleaned with piranha solution (concentrated sulfuric acid (v):hydrogen peroxide (v)=7:3) for 15 minutes, then ultrasonically cleaned with ethanol solution for 15 minutes, and finally cleaned with ultrapure water for 15 m...
Embodiment 2
[0043] Weigh 5.0 mg / mL of Fmoc-Tyr-OH solid powder, dissolve it in 100 μL of dimethyl sulfoxide, and ultrasonically disperse it for 30 seconds to completely dissolve it, and prepare a Fmoc-Tyr-OH stock solution with a concentration of 100 mg / mL; weigh Take 16.0 mg of amygdalin in a centrifuge tube, add 1.0 mL of ultrapure water to dissolve it, and prepare a stock solution of amygdalin with a concentration of 16 mg / mL.
[0044] Take 30μL of Fmoc-Tyr-OH stock solution and add it to a washed and dried 5.0mL screw bottle, add 950μL of amygdalin stock solution, shake it up quickly, and let it stand for 3 minutes to form a clear and transparent tyrosine-loaded amygdalin Hydrogel of amygdalin.
[0045] Fourier transform infrared test experimental procedure: After drying the sample in a freeze dryer for 12 hours, weigh 0.2g of potassium bromide powder and then add 2mg of the sample, put it into a mortar and grind it, mix the two thoroughly, and then put The mixture is put into a mold...
Embodiment 3
[0047] Weigh 5.0 mg / mL Fmoc-Tyr-OH solid powder, dissolve it in 100 μL dimethyl sulfoxide, and disperse it ultrasonically for 60 seconds to completely dissolve it to prepare a Fmoc-Tyr-OH stock solution with a concentration of 75 mg / mL; weigh Take 1.0 mg of amygdalin in a centrifuge tube, add 1.0 mL of ultrapure water to dissolve it, and prepare a stock solution of amygdalin with a concentration of 1 mg / mL.
[0048] Take 40μL of Fmoc-Tyr-OH stock solution and add it to a cleaned and dried 5.0mL screw bottle, add 960μL of amygdalin stock solution, shake it up quickly, and let it stand for 3 minutes to form a clear and transparent tyrosine-loaded amygdalin Amygdalin hydrogel.
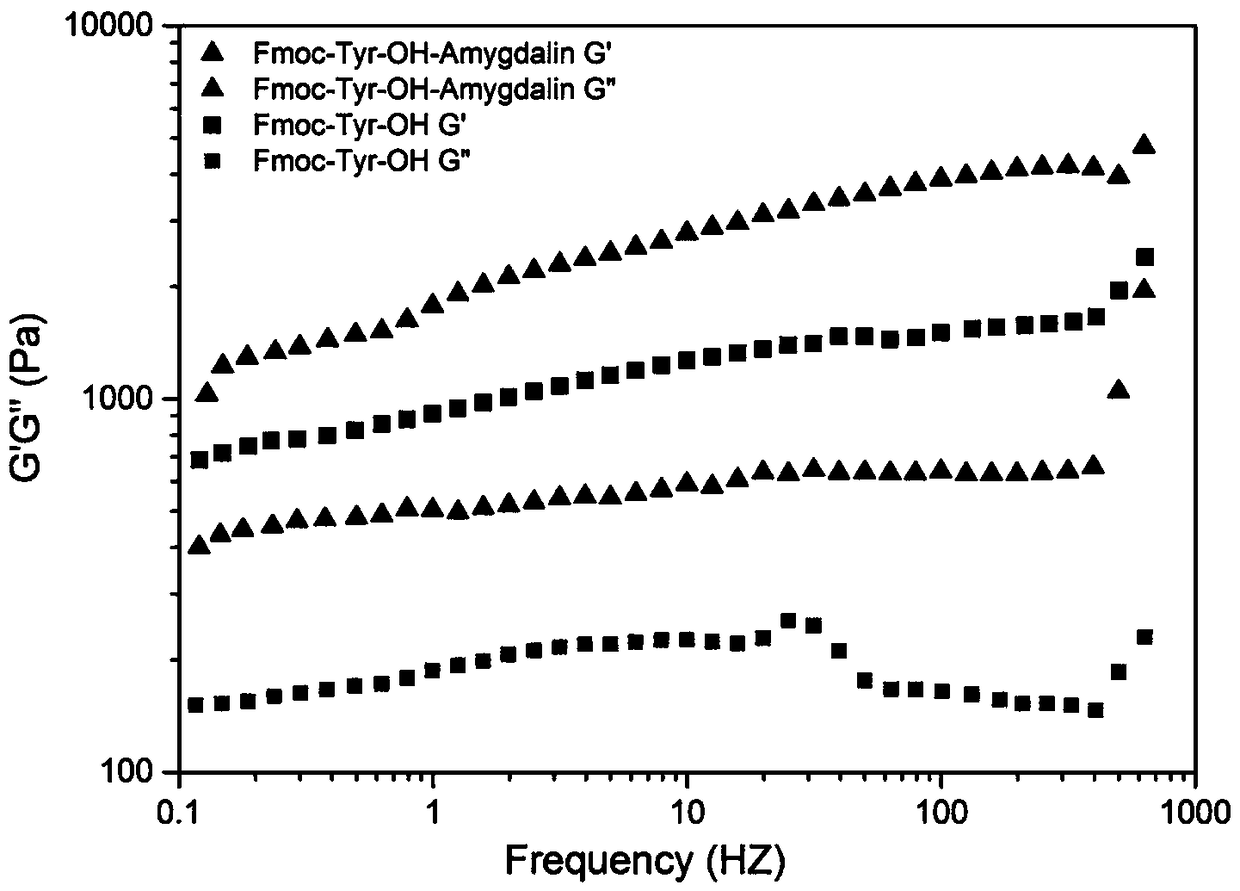
[0049] Rheological test experimental procedure: use AR2000 rheological instrument to test its rheological behavior, use a parallel plate with a diameter of 50mm, and set the gap to 0.2mm, test conditions: temperature is 25°C, intensity is 2%, frequency is 0.1— 100Hz.
[0050] Test results such as Figu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com