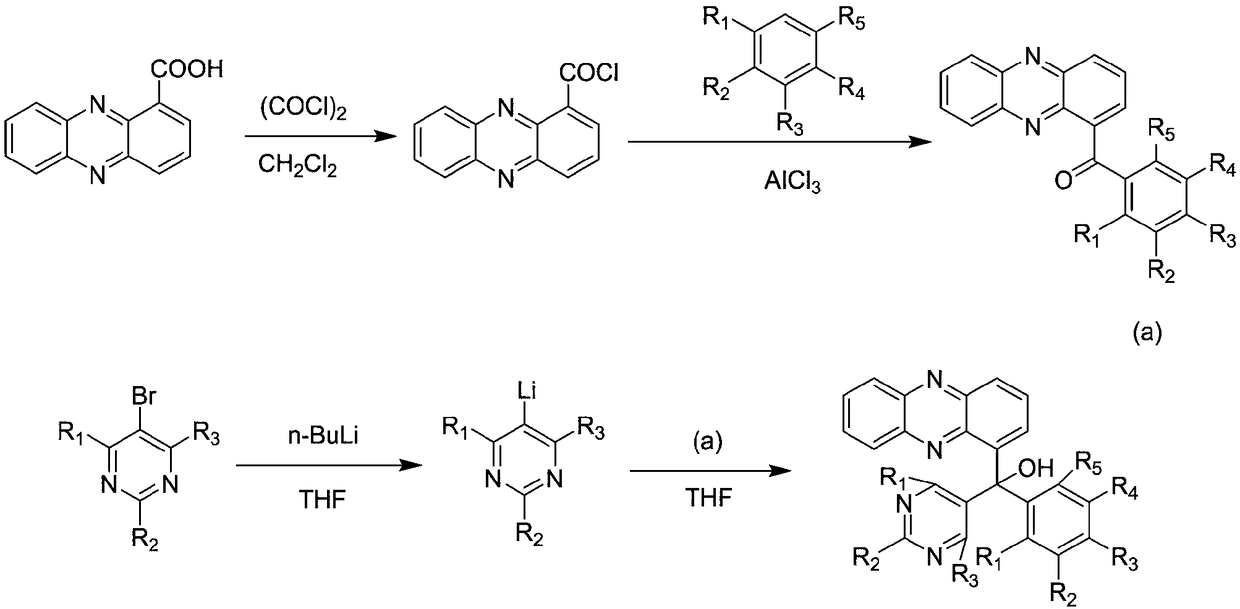
1-phenazinyl(phenyl)(5-pyrimidyl)methanol compound and preparation method and application thereof
A compound, phenazine-based technology, applied in the field of 1-phenazinyl carbinol compound and its preparation, can solve the problems of poor solubility and difficulty in dosage form processing, and achieve good antifungal effect, good solubility and good bactericidal activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
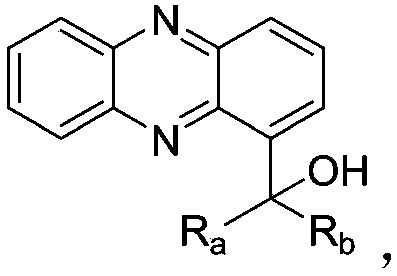
[0046] Example 1 Synthesis of 1-phenazinyl (phenyl) (5-pyrimidinyl) methanol
[0047] 1. Synthesis of phenazinyl-1-formyl chloride:
[0048]
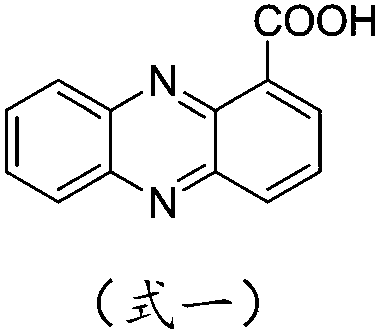
[0049] Add 2.5 g (11.2 mmol) of phenazinyl-1-carboxylic acid, 30 ml of dichloromethane into a 100 ml single-port reaction flask, drop 1 to 2 drops of DMF, slowly add 3.0 g of oxalyl chloride (to prevent flushing), and then heat to reflux Reaction, until the solid of Shenzimycin completely disappears, continue the reflux reaction for 2 to 3 hours, remove the solvent on a rotary evaporator, add a small amount of dichloromethane to dissolve, and then spin dry, try to take away the excess oxalyl chloride, For the next step.
[0050] 2. Synthesis of phenazin-1-yl (phenyl) ketone:
[0051]
[0052] Add 30ml of anhydrous benzene solution into a single-necked bottle containing phenazinyl-1-formyl chloride, and slowly add 8.4g (22.4mmol) of anhydrous AlCl 3 , reacted at room temperature for 7 hours, and monitored by pointing plate, the ...
Embodiment 2
[0061] Embodiment 2: Synthesis of phenazin-1-yl (4-phenoxy) (5-pyrimidinyl) methanol:
[0062] 1. Synthesis of phenazinyl-1-formyl chloride:
[0063]
[0064] Add 2.5 g (11.2 mmol) of phenazinyl-1-carboxylic acid, 30 ml of dichloromethane into a 100 ml single-port reaction flask, drop 1 to 2 drops of DMF, slowly add 3.0 g of oxalyl chloride (to prevent flushing), and then heat to reflux Reaction, until the solid of Shenzimycin completely disappears, continue the reflux reaction for 2 to 3 hours, remove the solvent on a rotary evaporator, add a small amount of dichloromethane to dissolve, and then spin dry, try to take away the excess oxalyl chloride, For the next step.
[0065] 2. Synthesis of phenazin-1-yl (4-phenoxyphenyl) ketone:
[0066]
[0067] Add 30ml of anhydrous diphenyl ether solution into a single-necked bottle containing phenazinyl-1-formyl chloride, and slowly add 3.03g (22.6mmol) of anhydrous AlCl 3 , reacted at room temperature for 8h, and monitored by...
Embodiment 3
[0076] Embodiment 3: Synthesis of (2,3-dimethylphenyl) (phenazin-1-yl) (5-pyrimidinyl) methanol
[0077] 1. Synthesis of phenazinyl-1-formyl chloride:
[0078]
[0079] Add 2.5 g (11.2 mmol) of phenazinyl-1-carboxylic acid, 30 ml of dichloromethane into a 100 ml single-port reaction flask, drop 1 to 2 drops of DMF, slowly add 3.0 g of oxalyl chloride (to prevent flushing), and then heat to reflux Reaction, until the solid of Shenzimycin completely disappears, continue the reflux reaction for 2 to 3 hours, remove the solvent on a rotary evaporator, add a small amount of dichloromethane to dissolve, and then spin dry, try to take away the excess oxalyl chloride, For the next step.
[0080] 2. Synthesis of (2,3-dimethylphenyl)(phenazin-1-yl)methanone:
[0081]
[0082] Add 30ml of anhydrous o-xylene solution into a single-necked bottle equipped with phenazinyl-1-formyl chloride, and slowly add 3.0g (22.6mmol) of anhydrous AlCl 3 , reacted for 12h under ice-bath condition...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com