Polypeptide capable of specifically binding EGFR for inhibiting EGF-promoting tumor cell proliferation
A tumor cell proliferation and tumor cell technology, applied in anti-tumor drugs, peptides, drug combinations, etc., can solve the problems of small molecule inhibitors are not as specific as monoclonal antibodies, patients cannot afford high costs for a long time, and the price is expensive. To achieve the effect of easy large-scale production, low production cost and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
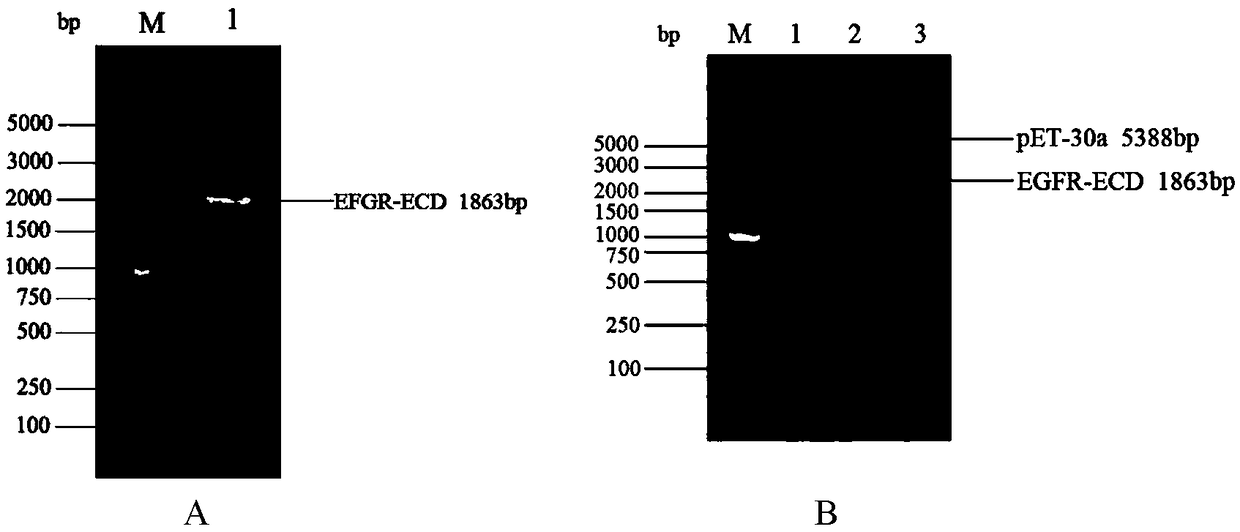
[0024] Embodiment 1: Construction of pET-30a / EGFR-ECD protein expression vector
[0025] Obtain the sequence information of EGFR from Genebank, accession number is CCDS5514.1, a total of 3363bp. EGFR extracellular domain (Extracellular Domain) is one of them (aa25-645), a total of 1863bp, extract the DNA sequence encoding EGFR-ECD fragment from the entire sequence of EGFR, as shown in SEQ.ID.NO.2, and design PCR based on it Primers:
[0026] Forward primer (SEQ.ID.NO.3):
[0027] 5'-GCTGATATC GGATCC ATGCTGGAGGAAAAGAAAGT-3'
[0028] and reverse primer (SEQ.ID.NO.4):
[0029] 5'-ACGGAGCTC GAATTCTCAGGACGGGATCTT AGG-3', the underlined parts are the restriction sites Bam HI and Eco RI at both ends respectively. The PCR product of EGFR-ECD and vector pET-30a (Novagen, #69909-3) were digested with Bam HI and Eco RI at 37 °C for 1 h, then ligated with T4 DNA ligase at 16 °C for 12 h. Transform the ligation product into DH5α competent cells (full gold, CD201), wait for a singl...
Embodiment 2
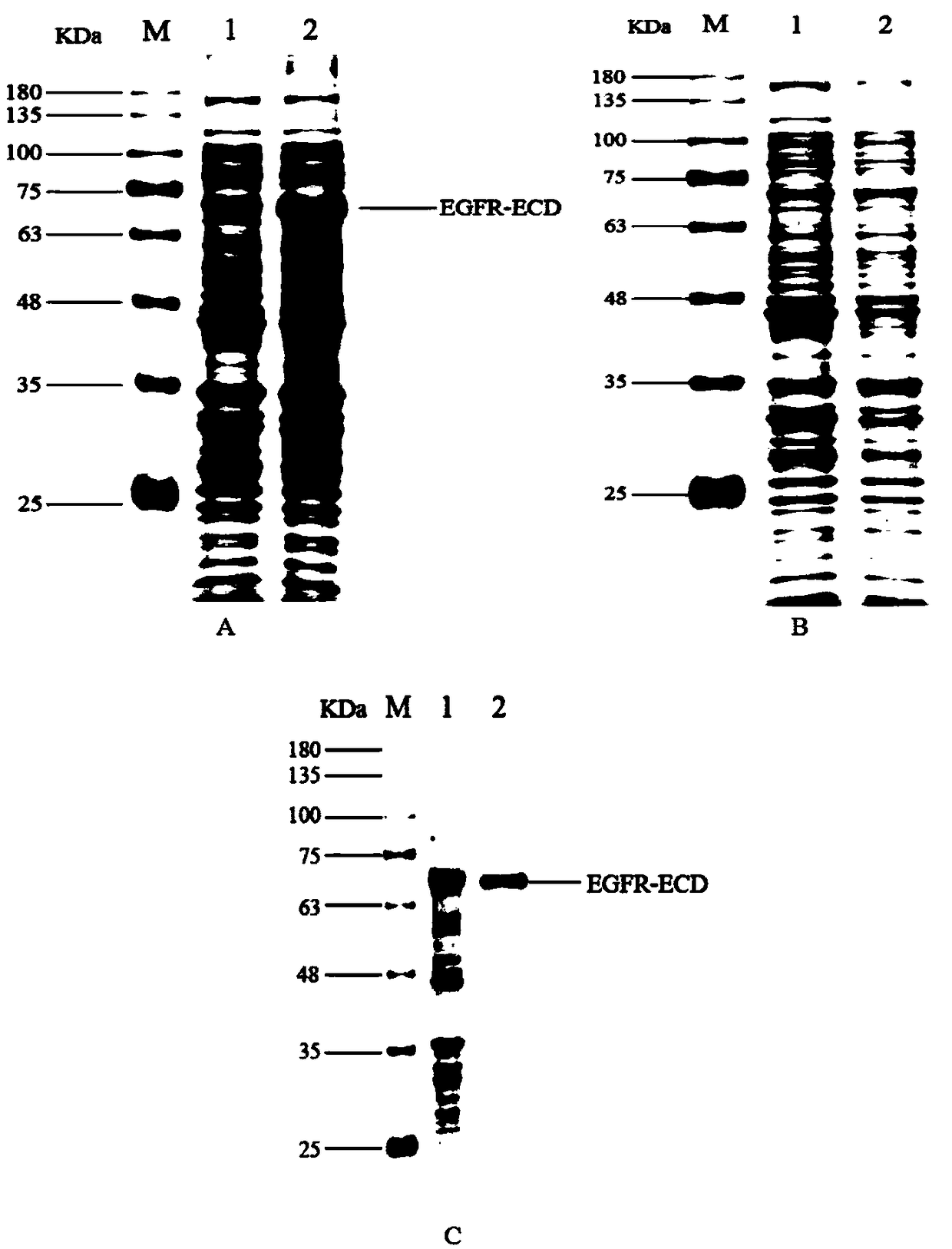
[0031] Example 2: Induced expression and refolding purification of EGFR-ECD
[0032] Induced expression of EGFR-ECD: Transform the successfully constructed recombinant plasmid pET-30a / EGFR-ECD into the host strain BL21(DE3) (full gold), use the kanamycin resistance plate to screen the recombinants, pick a single colony in Cultured in LB liquid medium containing kanamycin for 10 h. The culture was inoculated into LB liquid medium at a volume ratio of 1:100, and cultured with vigorous shaking at 37 °C until OD 600 =0.5~0.6, add 0.1 M IPTG to make the final concentration 0.5 mM, and induce at 25 °C for 4 h.
[0033] Verification of the expression of the target protein: centrifuge the above-mentioned induced bacterial solution at 5000 rpm for 10 min, remove the supernatant, and resuspend the bacteria in the lysate (50 mM Tris- HCl pH7.5, 500 mMNaCl, 10% glycerol, 1% TritonX-100, 1 mM protease inhibitor PMSF, 1 mg / mL lysozyme), put on ice and sonicate at 200 W power for 3 s wit...
Embodiment 3
[0038] Example 3: Phage display panning for biologically active peptides specifically binding to EGFR
[0039] Immobilization of target molecules: Add 100 µL of target molecule EGFR-ECD protein solution (0.1 M TBS pH7.4) at a concentration of 100 µg / mL into a 96-well plate, place on a shaker and incubate at 4 °C in a humidified container overnight. The target molecule solution was removed and washed 6 times with TBST (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% [v / v] Tween-20). Finally, block solution (0.1 M NaHCO 3 pH 8.6, 5 mg / mL BSA, 0.02% NaN 3 ) closed for 1 h.
[0040] Binding of phage random peptide library to target molecules: Remove the blocking solution and wash 10 times with TBST (containing 0.1% [v / v] Tween-20). Phage library (NEB (Beijing) Co., Ltd., Ph.D.-7 Phage Display Peptide Library Kit, Cat. No. #E8100S) or amplified phage diluted with TBST (containing 0.1% [v / v] Tween-20) , the titer of phage was 10 9 ~10 11 In between, the diluted phage was added to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com