Lipase mutant with improved thermal stability and application of lipase mutant
A lipase and mutant technology, applied in the fields of application, enzyme, hydrolase, etc., can solve the problems of unsatisfactory industrial production, poor heat resistance of Rhizopus sinica lipase, etc., and achieve the effect of reducing loss rate and improving heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of embodiment 1 lipase mutant
[0050] like figure 1 Shown, the obtaining method of the lipase mutant of the embodiment of the present invention comprises the following steps:
[0051] (1) Site-directed mutagenesis: the recombinant plasmid in which the Rhizopus sinensis lipase gene was linked to the carrier was used as a template, and the PCR amplification system for mutation was prepared according to the kit instructions to prepare a 50 μL mutation system.
[0052] (2) PCR verification of the mutation: 10 μL of the mutation product was taken and detected by 0.8% agarose gel electrophoresis. After the band is correct, add 1μDMT digestive enzyme to the mutant product, flick and mix well, and incubate at 37°C for 70min.
[0053] (3) Transformation: Add 5 μL of the mutated product to 50 μL DMT competent cells, flick and mix well, and place in ice bath for 30 min; heat shock at 42°C for 45 s and immediately cool on ice for 10 min; add 500 μL LB medium, 180 Tr...
Embodiment 2
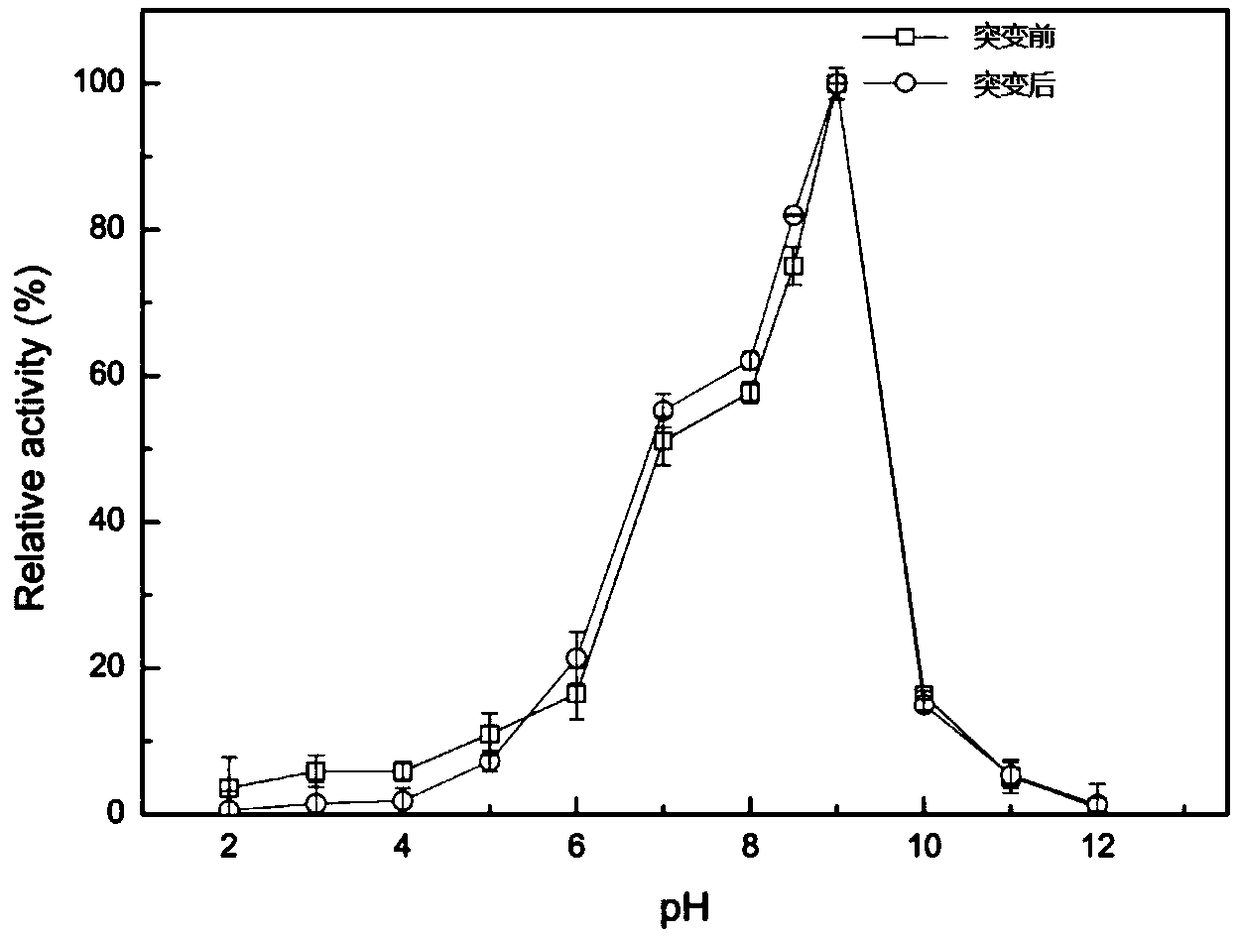
[0060] Embodiment 2 lipase optimum pH is measured
[0061] Adjust the pH of the buffer solution to 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, dilute the enzyme solution to an appropriate multiple, and measure the optimum pH at 37°C according to the lipase activity assay method. After the pH, add half points on both sides of the maximum value and continue to detect the optimum value (for example, if the optimum pH is 9, then take pH8, 8.5, 9, 9.5 and 10 to measure according to the lipase activity assay method).
[0062] The optimal pH value of the lipase enzymatic reaction is as follows: figure 2 shown. The optimum of mutant and lipase is 9. No significant changes.
Embodiment 3
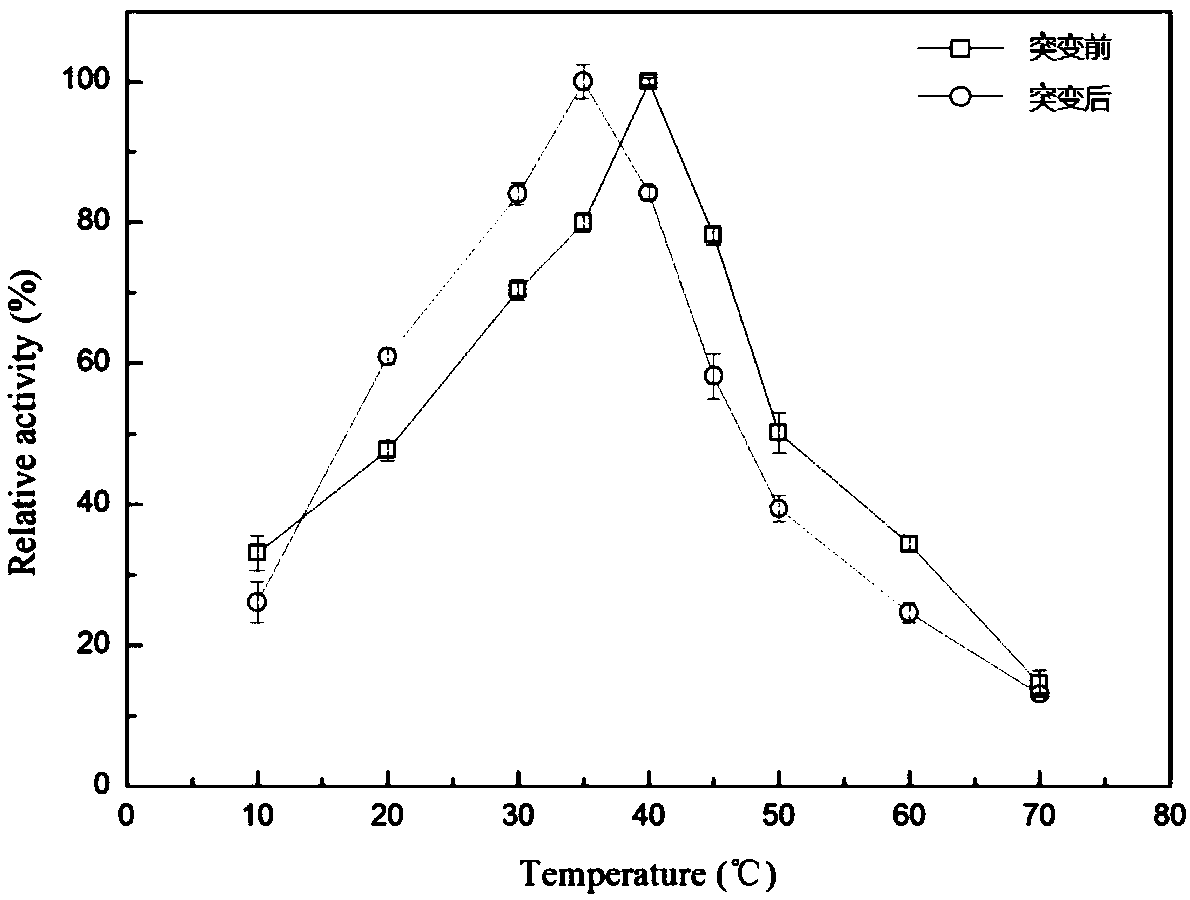
[0063] Embodiment 3 lipase optimum temperature is measured
[0064] According to the assay method of lipase activity, under the conditions of the above-mentioned optimum pH, the reactants were placed at different temperatures to react at 0°C, 10°C, 20°C, 30°C, 40°C, 50°C, 60°C, 70°C, 80°C, after measuring the optimum temperature, add half points on both sides of the maximum value (for example, if the optimum temperature is 40°C, add 30°C, 35°C, 40°C, 45°C, 50°C to determine the lipase activity method) .
[0065] Optimum temperature for lipase-catalyzed reaction image 3 As shown, the optimum temperature of the mutant and lipase were 35°C and 40°C respectively; the optimum temperature of the mutant was 5°C lower than that of the lipase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com