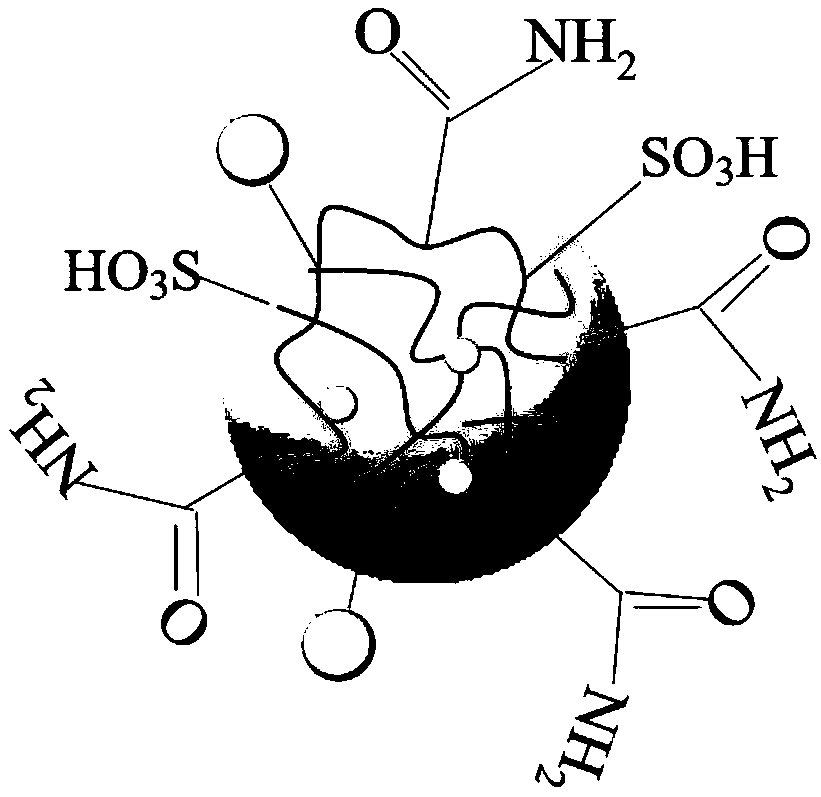
Heavy metal ion detection and adsorption integration polyacrylamide microgel
A technology of heavy metal ions and acrylamide, applied in the directions of alkali metal compounds, adsorbed water/sewage treatment, alkali metal oxides/hydroxides, etc., can solve the problems of temperature and speed of stirring, and achieve significant selectivity. , the effect of promoting absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
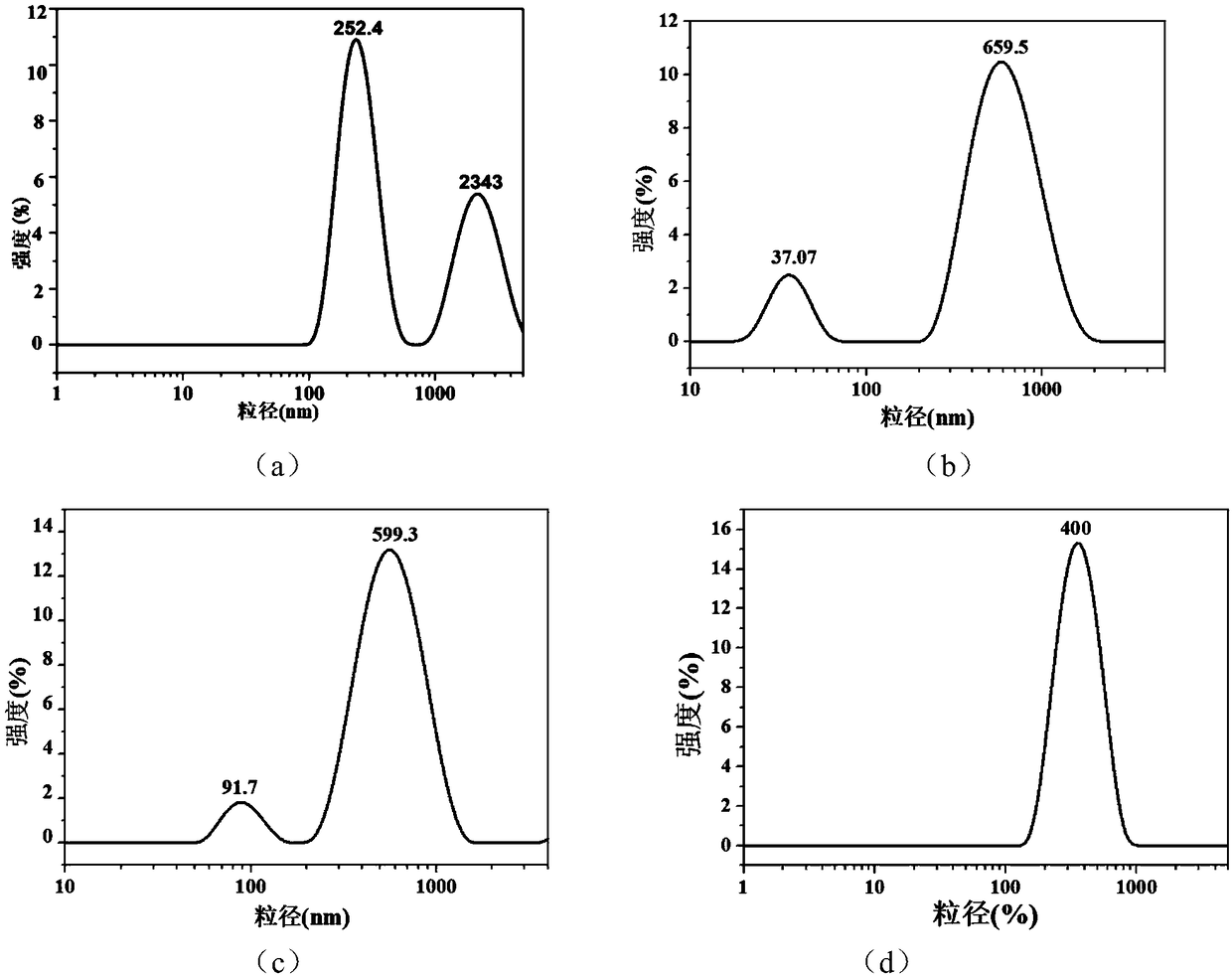
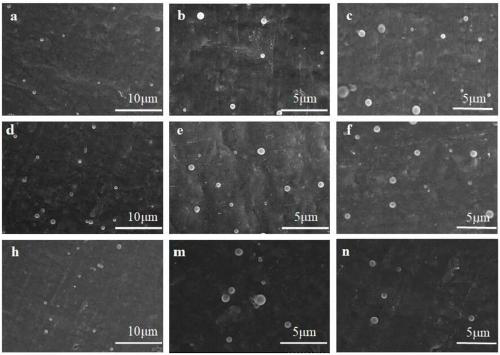
[0043] Embodiment 1 polyacrylamide microgel p(AM-CD-AMPS)
[0044] (1) Preparation of CDs: Weigh 0.48g of ethylenediamine and 0.46g of citric acid monohydrate, add them to a 50mL reaction kettle with a polytetrafluoroethylene lining, then add 10mL of ultrapure water, and ultrasonically 20- After 60 minutes, the reaction kettle was transferred to an oven at 100-300°C for 3-6 hours, and then cooled to room temperature naturally. The reacted solution was centrifuged to remove insoluble particles. After adjusting the above supernatant to pH ≈ 14.00 with a certain amount of NaOH solution, dry the solution to obtain a solid, then dissolve the solid in an appropriate amount of absolute ethanol, centrifuge to remove insoluble particles, and put the obtained carbon dot aqueous solution into Refrigerate in a refrigerator at 4°C for 1 day, and then filter with a 0.22 μm filter membrane to obtain a filtrate. The filtrate was freeze-dried to finally obtain carbon dots.
[0045] (2) Modi...
Embodiment 2
[0048] Embodiment 2 polyacrylamide microgel p(AM-CD-AMPS)
[0049] (1) Preparation of CDs: Weigh 0.96g of ethylenediamine and 0.92g of citric acid monohydrate, add them into a 50mL reaction kettle with a polytetrafluoroethylene liner, add 20mL of ultrapure water, and sonicate for 30min. The reaction kettle was transferred to an oven at 200° C. for 5 h, and then cooled to room temperature naturally. The reacted solution was centrifuged to remove insoluble particles. Use a certain amount of NaOH solution to adjust the above supernatant to pH ≈ 14.00, dry the solution to obtain a solid, then dissolve the solution in an appropriate amount of absolute ethanol, centrifuge to remove insoluble particles, and put the obtained carbon dot aqueous solution into Refrigerate in a refrigerator at 4°C for 1 day, and then filter with a 0.22 μm filter membrane to obtain a filtrate. The filtrate was freeze-dried to finally obtain carbon dots.
[0050] (2) Modification of CDs: Weigh 2 g of CDs...
Embodiment 3
[0053] Embodiment 3 polyacrylamide microgel p(AM-CD-AMPS)
[0054](1) Preparation of CDs: Weigh 1.44g of ethylenediamine and 1.38g of citric acid monohydrate, add them to a 50mL reaction kettle with a polytetrafluoroethylene liner, add 30mL of ultrapure water, and sonicate for 30min. The reaction kettle was transferred to an oven at 200° C. for 5 h, and then cooled to room temperature naturally. The reacted solution was centrifuged to remove insoluble particles. After adjusting the above supernatant to pH ≈ 14.00 with a certain amount of NaOH solution, dry the solution to obtain a solid, then dissolve the solid in an appropriate amount of absolute ethanol, centrifuge to remove insoluble particles, and put the obtained carbon dot aqueous solution into Refrigerate in a refrigerator at 4°C for 1 day, and then filter with a 0.22 μm filter membrane to obtain a filtrate. The filtrate was freeze-dried to finally obtain carbon dots.
[0055] (2) Modification of CDs: Weigh 3 g of CD...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com