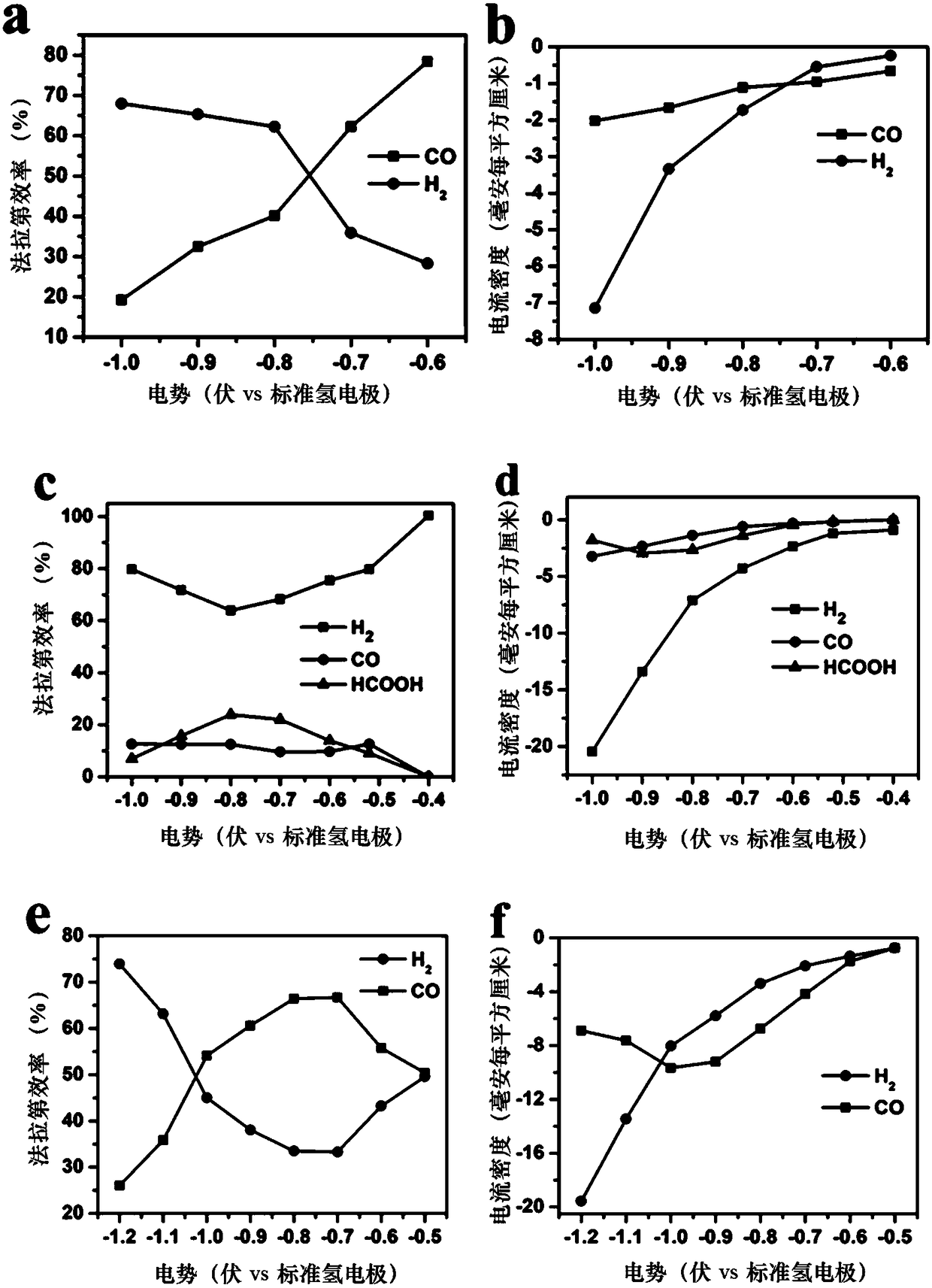
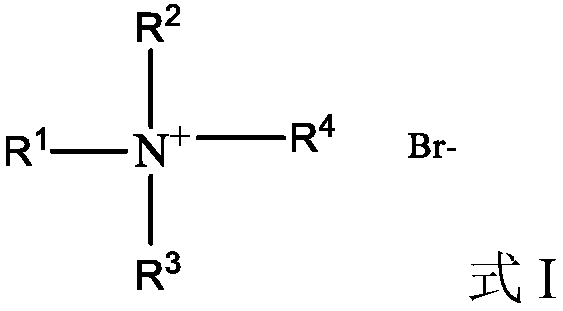Au-Ir nano alloy, preparation method of Au-Ir nanometer alloy and application of Au-Ir nanometer alloy used as catalyst
A nano-alloy and catalyst technology, applied in nanotechnology, nanotechnology, metal processing equipment, etc., can solve the problems of large difference in reduction potential and difficulty in obtaining controllable morphology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
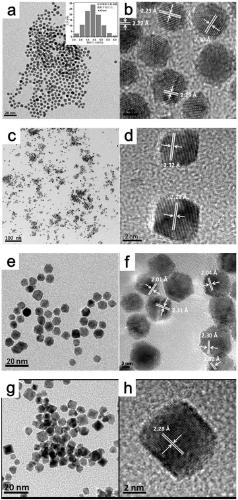
[0117] Embodiment 1: the preparation method of Au-Ir bimetallic nanoparticles
[0118] (1) Add 0.1 mmol of iridium chloride trihydrate and 0.1 mmol of tetrachloroauric acid trihydrate into a 100 ml two-necked flask, then add 35 ml of oleylamine and sonicate for 60 min to obtain mixture A.
[0119] (2) Put mixture A into an oil bath preheated to 200°C, N 2 Under the atmosphere, react for 1h to obtain the product B.
[0120] (3) Naturally cool to room temperature, centrifuge the obtained product B (12000 rpm), and wash 6 times with a mixed solution of ethanol and cyclohexane (the volume ratio of ethanol and cyclohexane is 1:1).
[0121] (4) drying at 70° C. overnight to obtain the Au-Ir bimetallic nanoparticles of the present invention.
Embodiment 2
[0122] Embodiment 2: the preparation method of Au-Ir double metal nano-octahedron
[0123] (1) 0.1mmol of iridium chloride trihydrate, 0.1mmol of tetrachloroauric acid trihydrate, 0.6mmol of tetraethylammonium bromide, 100mg of PVP (molecular weight is 40000) are joined in the 100ml two-necked flask, then add 35ml Oleylamine was sonicated for 60 min and stirred for 4 h to obtain mixture A.
[0124] (2) Put mixture A into an oil bath preheated to 220°C, N 2 Under the atmosphere, react for 1h to obtain the product B.
[0125] (3) Naturally cool to room temperature, centrifuge (12000 rpm) to obtain product B, water, ethanol, hexanaphthene and acetone mixed solution (the volume ratio of water, ethanol, hexanaphthene and acetone is 1:4:1 :2) Wash 14 times.
[0126] (4) drying at 70° C. overnight to obtain the Au-Ir bimetallic nano-octahedron of the present invention.
Embodiment 3
[0127] Embodiment 3: the preparation method of Au-Ir bimetallic nano-truncated octahedron
[0128] (1) 0.1mmol of hydrated hexachloroiridium (IV) acid, 0.1mmol of tetrachloroauric acid trihydrate, 0.6mmol of tetraethylammonium bromide, and 33.3mg of PVP (molecular weight is 55000) are added in a 100ml two-necked flask , then add 30ml oleylamine and sonicate for 60min, stir for 4h to obtain mixture A.
[0129] (2) Put mixture A into an oil bath preheated to 220°C, N 2 Under the atmosphere, react for 1h to obtain the product B.
[0130] (3) naturally cool to room temperature, obtain product B centrifugation (12000 rpm), water, ethanol, cyclohexane and acetone mixed solution (the volume ratio of ethanol, ether, normal hexane and acetone is 5:5:3: 5) Wash 12 times.
[0131] (4) drying at 70° C. overnight to obtain the Au-Ir bimetallic nano-truncated octahedra of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com