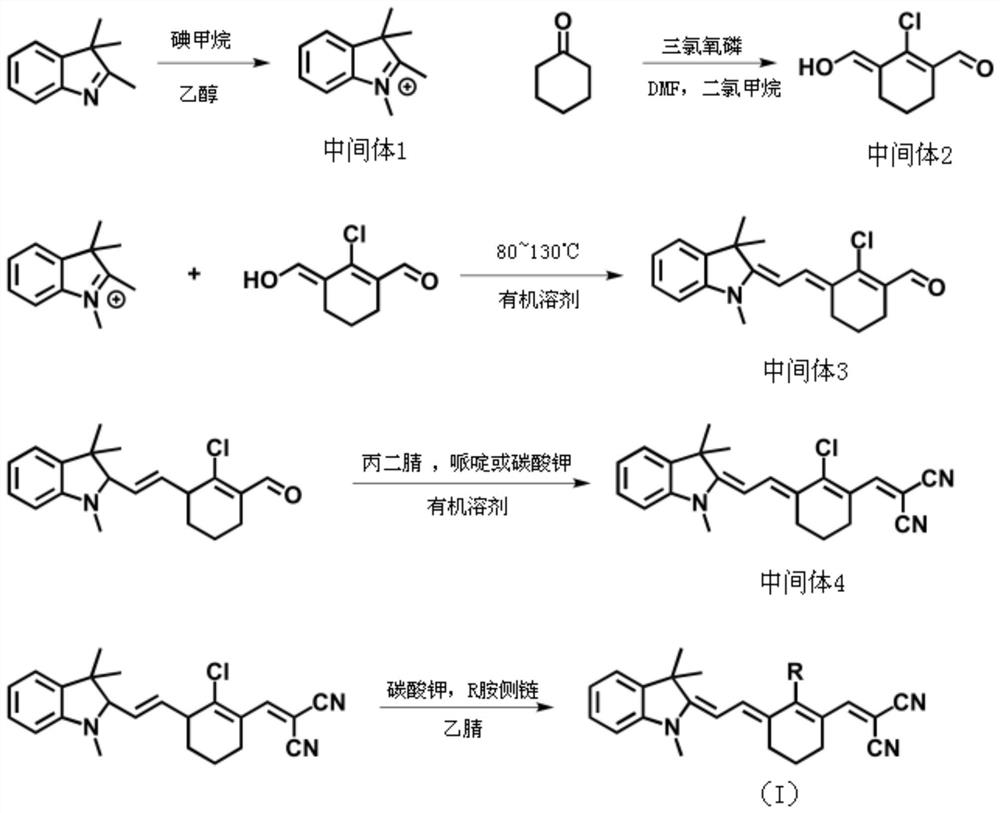
A kind of merocyanine fluorescent compound and its preparation method and application
A technology of fluorescent compounds and cyanines, applied in chemical instruments and methods, organic chemistry, pharmaceutical formulations, etc., can solve problems such as limited, unavailable confirmatory diagnosis, and high cost, and achieve low background fluorescence interference and enhanced fluorescence quantum yield , low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthesis of a merocyanine fluorescent compound of the present embodiment, the specific synthesis steps are as follows:
[0039] (1) Synthesis of Intermediate 1
[0040] Stir 4.77g of 2,3,3-trimethylindole (30mmol) and 3.22ml of iodomethane (7.08g, 50mmol) in 60ml of ethanol, seal the pressure vessel, and react at 80°C for 12h. After the reaction was completed, it was cooled to room temperature, and the solid was precipitated, and filtered by suction to obtain 7.7 g of the precipitated solid, namely the lavender intermediate 1, with a yield of 85%. NMR data are as follows: 1H NMR (400MHz, DMSO) δ7.92-7.86 (m, 1H), 7.85-7.78 (m, 1H), 7.66-7.58 (m, 2H), 3.96 (s, 3H), 2.76 (s ,3H), 1.52(s,6H).
[0041] (2) Synthesis of Intermediate 2
[0042]Mix 17.5ml of DMF (16.54g, 225mmol) and 18ml of dichloromethane evenly, in an ice bath and under the protection of nitrogen, slowly add 15ml of phosphorus oxychloride (24.68g, 160mmol) drop by drop, stir for 20-40min, then add ...
Embodiment 2
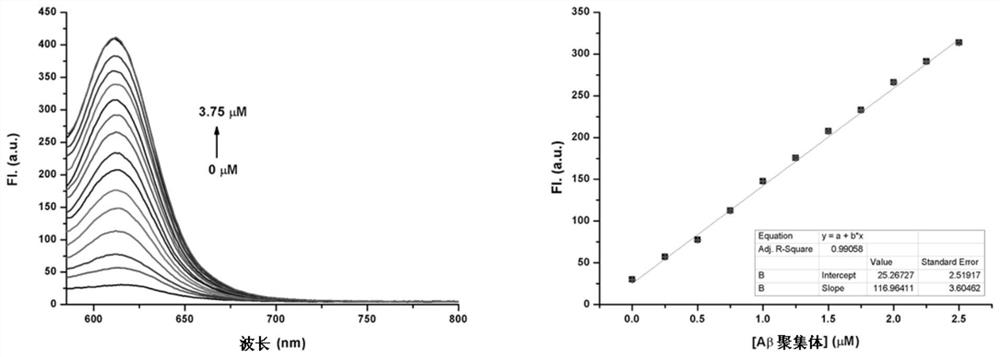
[0067] The maximum absorption wavelength (λ abs ) and maximum emission wavelength (λ em )experiment:
[0068] Experimental method: Fluorescent compounds D1-D9 were dissolved in dimethyl sulfoxide to prepare a 10 mM stock solution. Take 3 μL of the above solution and dilute to 3 mL with different polar organic solvents DCM, EtOH, ACN, MeOH, DMSO, PBS to obtain a 10 μM solution, and use a UV-2450, SHIMADZU and a fluorescence spectrophotometer (F-4500, Hitachi), the maximum absorption wavelength and maximum emission wavelength of the fluorescent compound were measured and recorded.
[0069] The fluorescence spectra of fluorescent compounds D9-D18 in different solvents in this example are shown in Table 1.
[0070] Table 1 The maximum absorption wavelength and emission wavelength of compounds D9~D18 in different solvents
[0071]
[0072]
[0073] It can be seen from Table 1 that the emission wavelength of the fluorescent compound of the present invention red shifts as t...
Embodiment 3
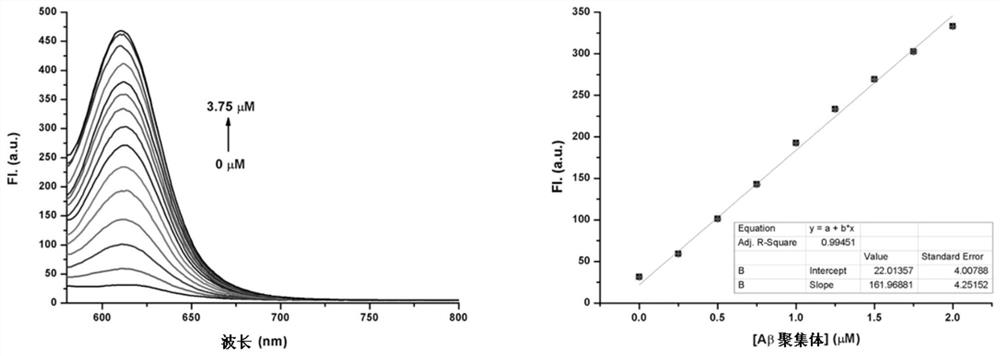
[0075] Fluorescence quantum yield measurement experiments of fluorescent compounds D1-D9 in DCM and PBS;
[0076] Experimental method: select cresyl violet acetate as the reference compound (in methanol, its fluorescence quantum yield is 0.54), and measure the fluorescent compounds D1-D9 on the UV-visible spectrophotometer and the fluorescence spectrophotometer respectively And the ultraviolet absorption spectrum and fluorescence emission spectrum of the reference compound, according to Y u =Y s *F u / F s *A s / A u Perform calculations to obtain the fluorescence quantum yield of the fluorescent compound. Y u , Y s is the fluorescence quantum yield of the substance to be tested and the reference standard substance, F u , F s is the integrated fluorescence intensity of the substance to be tested and the reference substance, A u 、A s is the incident light intensity of the test substance and the reference substance at the excitation wavelength.
[0077] The fluorescenc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com