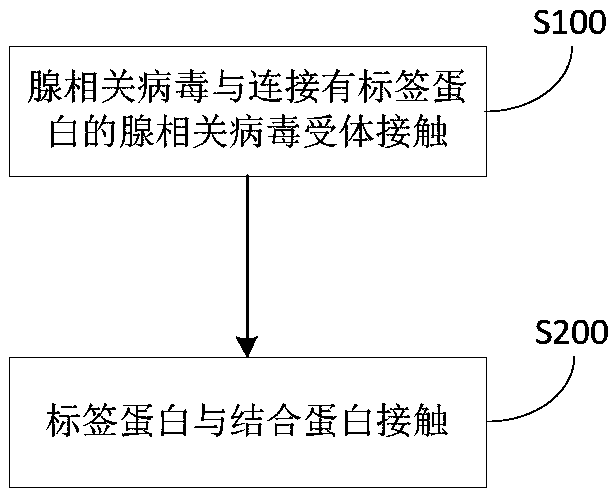
Method for treating adeno-associated viruses and kit
A virus and solid-phase carrier technology, applied in the biological field, can solve the problems of adeno-associated virus purification, identification and titer determination methods to be studied, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1. Obtain purified AAV virus particles from a small amount of crude virus extract by SAV-AAVR-SBP magnetic bead method
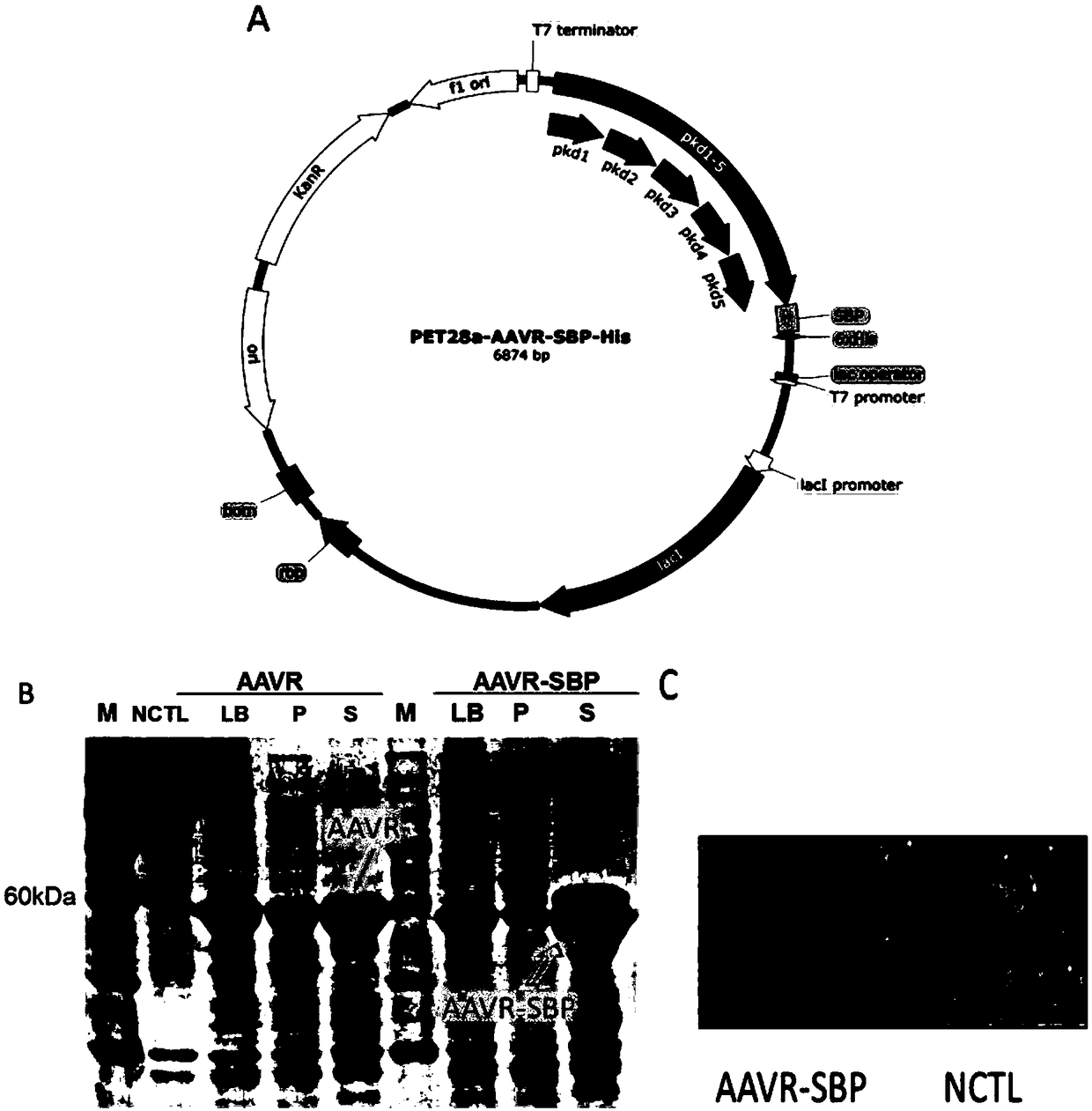
[0067] 1.1 Expression and purification of AAVR-derived AAVR protein ( figure 2 )
[0068] The present invention firstly produces and purifies the protein of the PKD1-5 domain in the AAVR protein sequence (referred to as AAVR protein), and carries a His tag and a biotin-affinity SBP tag at the C-terminal of the AAVR protein.
[0069] 1) PET-28a-AAVR and PET-28a-AAVR-SBP fusion plasmids were constructed using PET-28a as a vector.
[0070] 2) The two fusion plasmids were transformed with 100 μL of BL21 competent cells as host bacteria, and cultured at 37°C for 14 hours.
[0071] 3) Pick a monoclonal colony, inoculate it in 5 ml of kanamycin-resistant liquid LB, shake the bacteria at 37°C at 220 rpm for 8 hours, and inoculate it in 1 L of kanamycin-resistant liquid LB medium at a ratio of 1:1000, under the same conditions Cultivate until the OD value ...
Embodiment 2
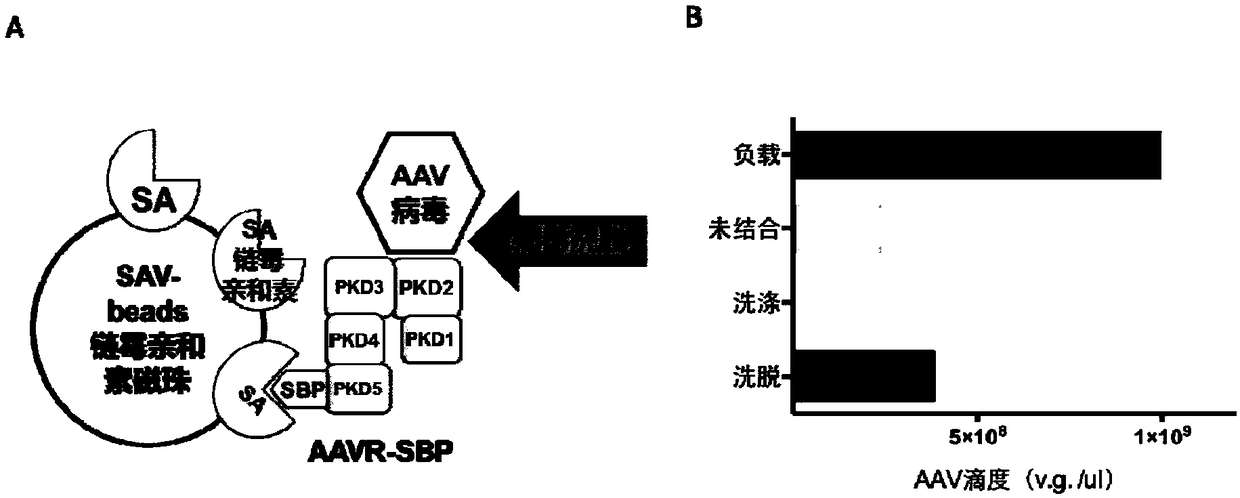
[0092] 2. Purified AAV virus particles were obtained from a large number of crude virus extracts by AAVR-SAV prepacked column chromatography ( Figure 4 )
[0093] Figure 4 A shows the schematic diagram of the reaction flow, using SAV as the solid carrier to load the AAVR-SBP protein. After immobilizing the PKD protein, the crude extract containing AAV particles was passed through the prepacked column, and the AAV was bound to the AAVR-SBP protein and eluted with a high salt solution to obtain the AAV virus.
[0094] 2.1 Preparation of AAVR-SAV prepacked column
[0095] A 5 ml streptavidin agarose (SAV) prepacked column (ThermoFisher Company, USA) was used, the loading buffer was 20 mM Tris-HCl, pH=7.4, and the loading flow rate was 1 ml / min. The loading buffer was equilibrated for 5 times the column volume, and a total of 5 ml of 1 mg / ml AAVR-SBP protein solution (obtained in step 1.1 of Example 1) was loaded, and the flow-through was collected and loaded twice. Then, eq...
Embodiment 3
[0103] 3. The titer of purified AAV virus was identified by AAVR protein-coated ELISA kit ( Figure 5 )
[0104] Figure 5 A shows the schematic diagram of the kit. The AAVR protein is used as the capture antibody, and the AAV virus is added in a series of dilutions, and the AAVR-SBP protein is used as the primary antibody. SAV-HRP was used as secondary antibody, ABTS was used as HRP reaction substrate, and the degree of color development of each sample was determined by 405nm absorption light of microplate reader.
[0105] 3.1 Coating of AAVR protein
[0106] The AAVR protein was diluted to 1 mg / ml with coating diluent (0.05mol / L sodium carbonate-sodium bicarbonate buffer, pH 9.6). Add 100 μL of diluted AAVR protein to each well of the ELISA plate and incubate at 4°C overnight. Ensure the ambient humidity, complete the coating after overnight, and discard the liquid in the wells.
[0107] 3.2 ELISA plate blocking
[0108] Block with 5% calf serum at 37°C for 40min. When...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com