Electrochemical method for detecting activity of protein acetyltransferase
An acetyltransferase, electrochemical technology, applied in the direction of electrochemical variables of materials, measuring devices, scientific instruments, etc., can solve the problems of high cost of antibody labeling, complex probe preparation, differences between antibody batches, etc., and achieve a high degree of design. Flexibility, selectivity and repeatability, enhanced selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Technical solution and working principle
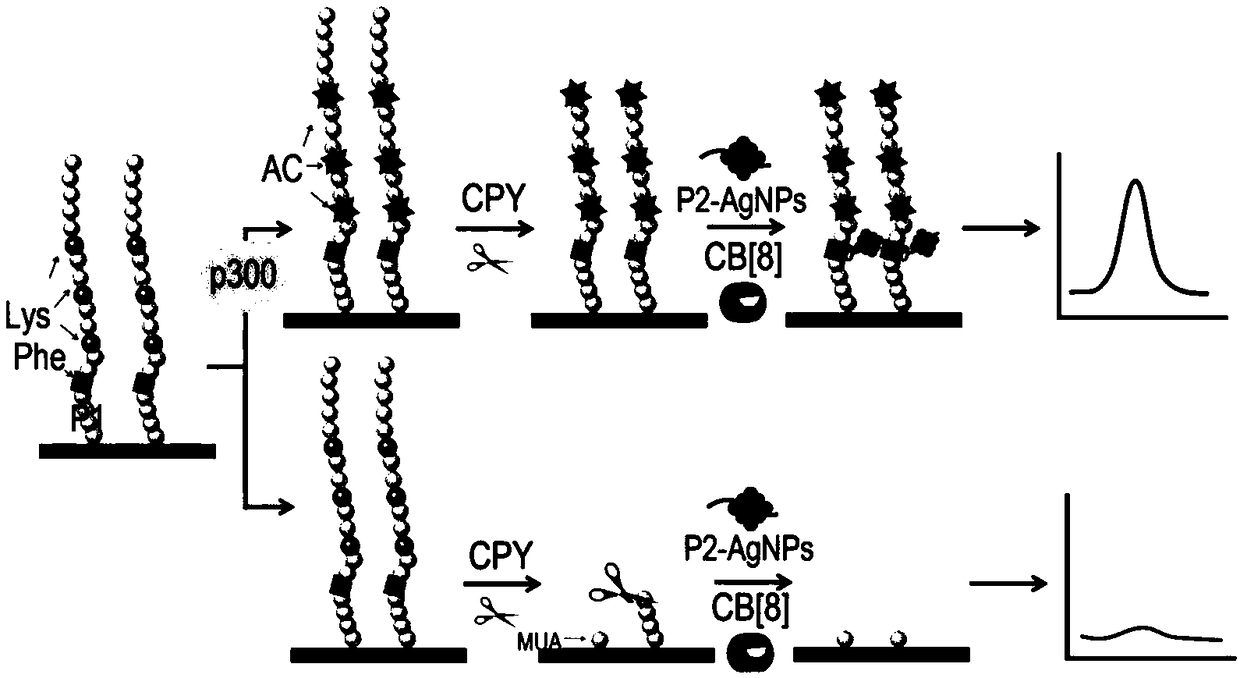
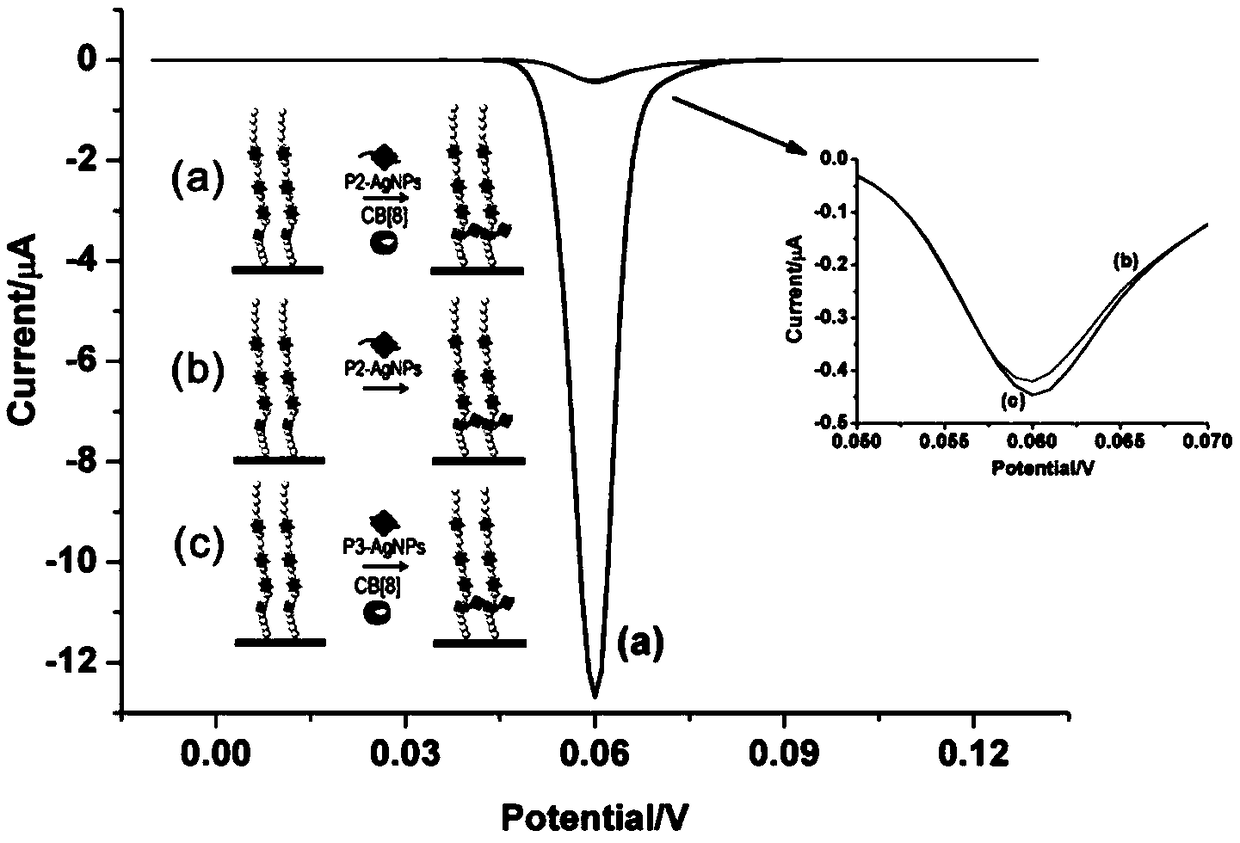
[0044] An electrochemical method for detecting protein acetyltransferase activity according to the present invention comprises the following steps: adding P2 templated silver nanoparticles (P2-AgNPs) to the detection system of P1 modified gold electrode (P1 / AuE) and p300 protein ) and cucurbit[8] urea (CB[8]), the activity of p300 protein was detected by electrochemical measurement, so as to obtain the activity of protein transferase; the amino acid sequence of P1 is: 11-mercaptoundecanoic acid (MUA) -GGGFRGKGGKGLGKGGAKA; the amino acid sequence of P2 is: FGGGASLWWSEKL.
[0045] The working principle of the present invention, such as figure 1 As shown, the present invention provides a polypeptide probe whose amino acid sequence is P1: 11-mercaptoundecanoic acid (MUA)-GGGFRGKGGKGLGKGGAKA, wherein cysteine C can realize the polypeptide probe on the surface of the gold electrode through the Au-S bond The self-assembly of...
Embodiment 2
[0047] Synthesis of P2-templated silver nanoparticles (P2-AgNPs)
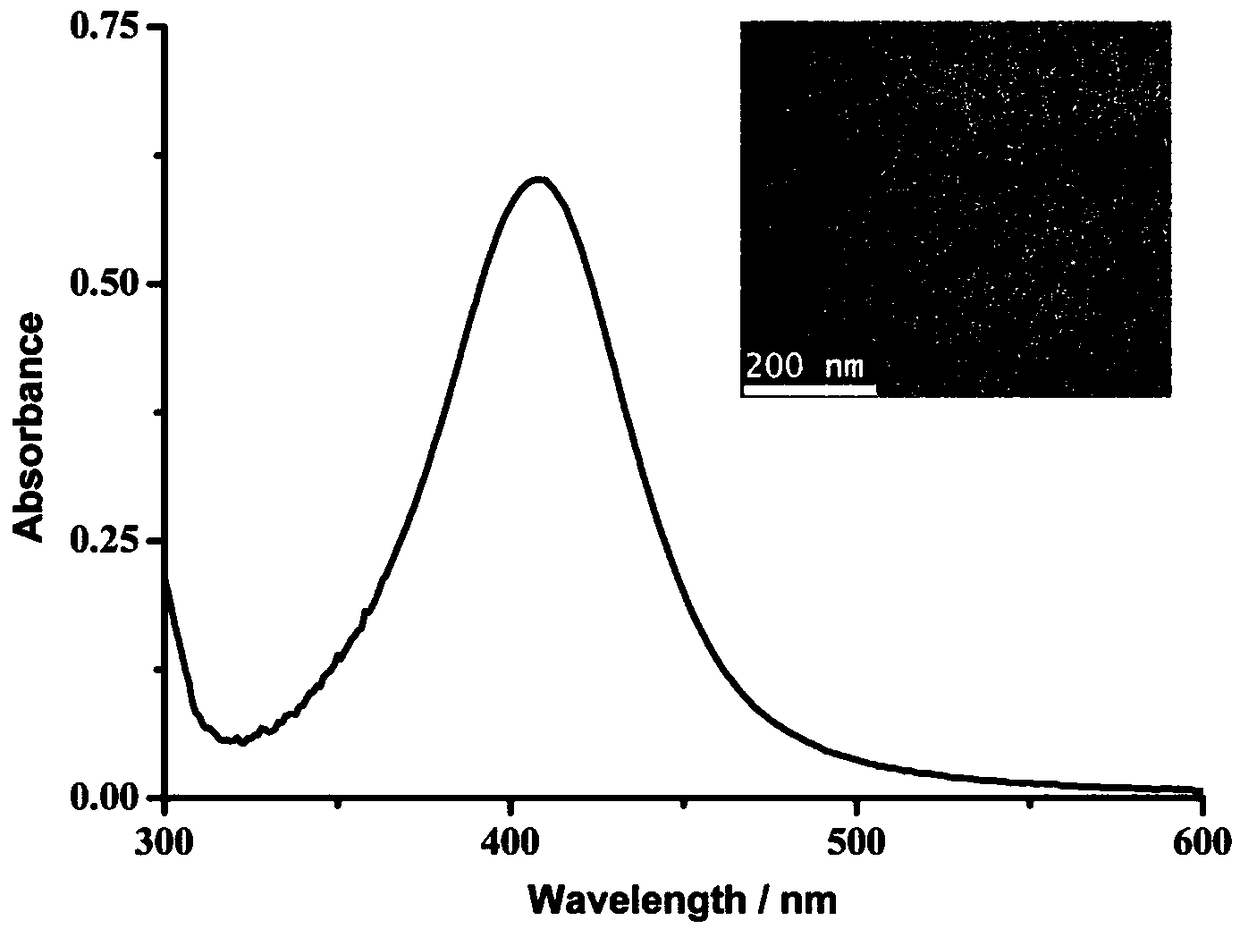
[0048] The synthesis method of P2-templated silver nanoparticles (P2-AgNPs), including the following steps: 0.2 mM AgNO 3 Mix with 100ng / μL P2 in 5mL aqueous solution, and magnetically stir for 20min. NaBH4 (2 mM) was slowly added to the mixed solution, and magnetically stirred for 10 min. The formation of P2-AgNPs was indicated when the final solution appeared a stable yellow color.
[0049] like figure 2 As shown, the formation and availability of P2-AgNPs were first investigated, and in the UV-vis spectra, the characteristic peak at 405 nm confirmed the presence of Ag nanoparticles. By TEM analysis, most of the particles are dispersed with a particle size of about 10 nm (inset).
[0050] In the control experiment, the control polypeptide P3 with the phenylalanine residue removed was used instead of P2 for template synthesis of silver nanoparticles. Wherein, the amino acid sequence of P3 is: GGGA...
Embodiment 3
[0053] Preparation of P1 modified gold electrode (P1 / AuE)
[0054] Before modification, the gold electrode first needs to be polished into a mirror surface with 1 μm, 0.3 μm and 0.05 μm alumina suspension on the suede. Then, with piranha solution (H 2 SO 4 : H 2 o 2 = 3: 1) Soak and wash for 5 minutes to remove organic matter adsorbed on the electrode. Electrodes were ultrasonically cleaned in double distilled water and ethanol, respectively. The electrode was soaked in 50% nitric acid for 30min, then in 0.5MH 2 SO 4 In electrochemical cleaning, the scanning voltage was from 0 V to 1.5 V, and the cycle was 30 times. Blow dry in a nitrogen atmosphere, and immerse the treated gold electrode in 100 μL, 0.5 μM P1 solution. Trichloroethyl phosphate (TCEP) was added to the P1 solution to prevent the MUA end groups of P1 from forming disulfide bonds. After soaking for 16 hours, the gold electrodes were incubated in 1 mM MCH for 30 minutes, washed with double distilled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com