Material for thioketal bonding doxorubicin and polyphosphate, and preparation method and application thereof
A technology of polyphosphate ester and adriamycin, which is applied to medical preparations without active ingredients, wave energy or particle radiation treatment materials, and medical preparations containing active ingredients, etc. Regional drug release, drug leakage and other problems, to achieve the effect of improving utilization rate and therapeutic effect, increasing drug concentration, and significant clinical application significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
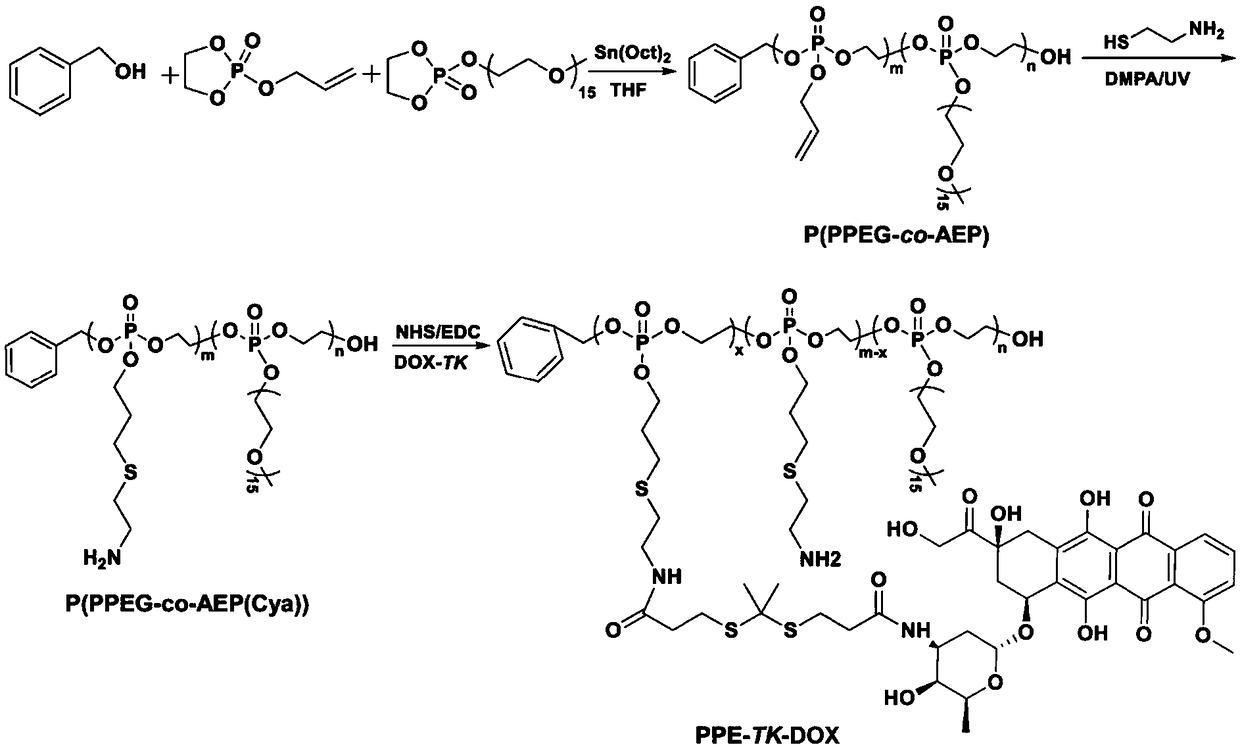
[0051] Example 1. Synthesis and characterization of reactive oxygen species-responsive polyphosphate-bonded doxorubicin PPE-TK-DOX
[0052] 1. Synthesis of reactive oxygen species-responsive polyphosphate-bonded doxorubicin PPE-TK-DOX
[0053] PEGylated phosphate monomer (PPEG) and 2-(allyloxy)-2-oxo-1,3,2-di Polyphosphate P (PPEG-co-AEP) was obtained by ring-opening polymerization of oxaphospholane (2-(allyloxy)-1,3,2-dioxaphospholane 2-oxide, AEP). Then react with mercaptoethylamine to obtain polyphosphate P (PPEG n -co-AEP(Cya) m ). 3,3'-(propane-2,2-diylbis(sulfadiyl))dipropionic acid was added to doxorubicin (DOX) under activated conditions for amidation reaction, and then polyphosphate P( PPEG n -co-AEP(Cya) m ) further amidation reaction, after finishing the reaction, the material with thioketal bonded doxorubicin and polyphosphate is obtained. For the synthesis steps, see figure 1 .
[0054] 1. Polyphosphate P (PPEG 10 -co-AEP(Cya) 20 )Synthesis
[0055] Bu...
Embodiment 2
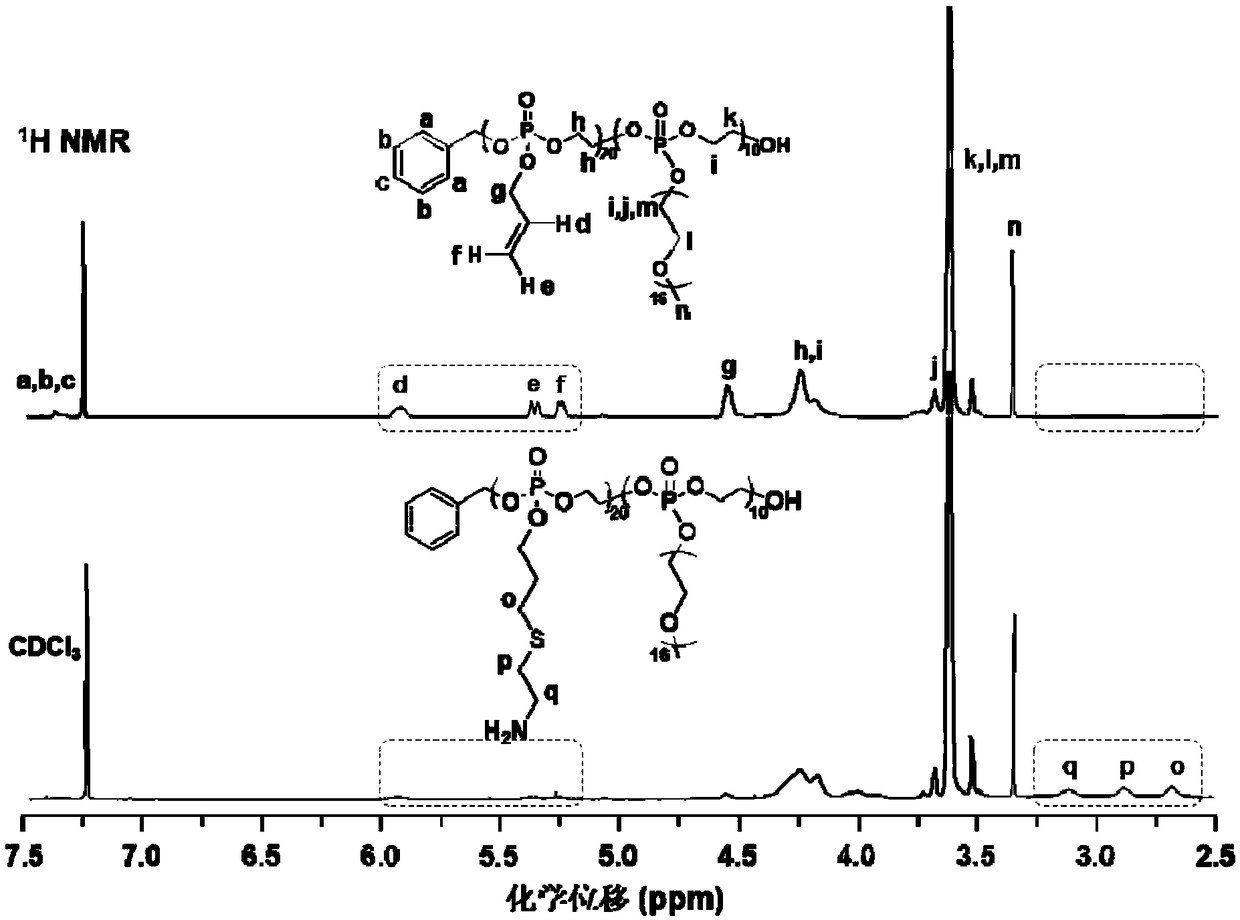
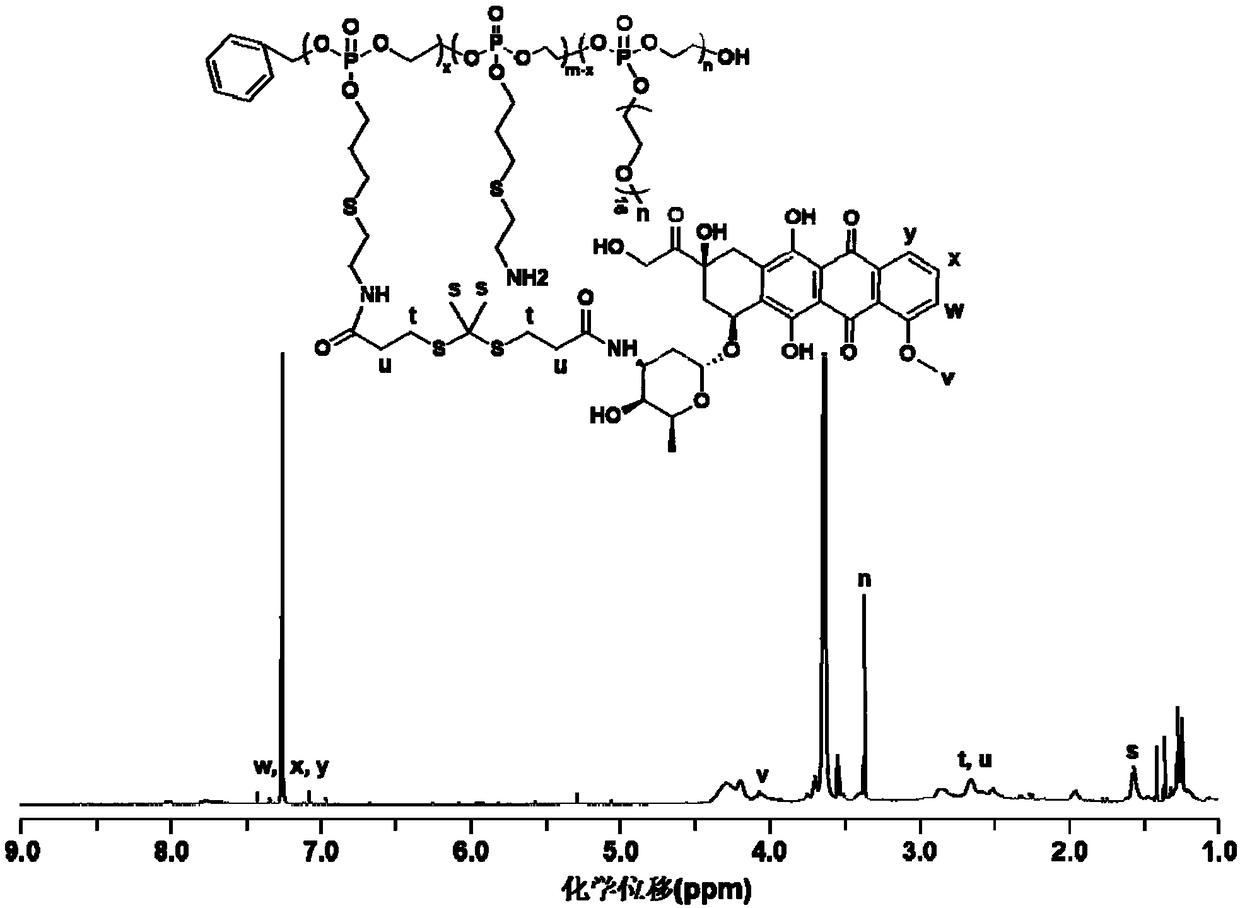
[0063] The material characterization result of embodiment 2 and embodiment 3 is similar to that of embodiment 1, can refer to the proton nuclear magnetic resonance spectrum of embodiment 1 ( 1 H NMR).
[0064] Example 2. Synthesis of active oxygen-responsive polyphosphate-bonded doxorubicin PPE-TK-DOX
[0065] 1. Polyphosphate P (PPEG 20 -co-AEP(Cya) 30 )Synthesis
[0066] Build a set of anhydrous reaction device to remove water vapor. In a glove box, AEP (0.62 g, 3.78 mmol), PPEG (1.65 g, 1.89 mmol), and benzyl alcohol (0.01 g, 0.092 mmol) and 8 mL of purified and dried tetrahydrofuran (THF) were added to an anhydrous, oxygen-free In the flask, after fully stirring at 45°C for 20 minutes, add stannous octoate (12 mg, 0.03 mmol) and react at 45°C for 2 d to complete the polymerization reaction. The product was precipitated twice in ether / methanol (4:1, v / v) to give P(PPEG n -co-AEP m ).
[0067] P(PPEG 20 -co-AEP 30 ) (500mg, 0.022mmol), mercaptoethylamine (225mg, 1....
Embodiment 3
[0070] Example 3. Synthesis of reactive oxygen species-responsive polyphosphate-bonded doxorubicin PPE-TK-DOX
[0071] 1. Polyphosphate P (PPEG) 15 -co-AEP(Cya) 25 )Synthesis
[0072] Build an anhydrous reaction device to remove water vapor. AEP (0.44 g, 2.7 mmol), PPEG (1.18 g, 1.35 mmol), and benzyl alcohol (0.01 g, 0.092 mmol) and 8 mL of purified and dried tetrahydrofuran (THF) were added to anhydrous and oxygen-free in a glove box. In the flask, after fully stirring at 43 °C for 20 min, stannous octoate (12 mg, 0.03 mmol) was added, and the reaction was carried out at 43 °C for 60 h to complete the polymerization reaction. The product was precipitated twice in ether / methanol (4:1, v / v) to give P(PPEG 15 -co-AEP 25 ).
[0073] P(PPEG 15 -co-AEP 25 ) (500 mg, 0.03 mmol), mercaptoethylamine (153 mg, 1.35 mmol) was dissolved in 4 mL of DMF, and 2,2-dimethoxy-2-phenylacetophenone (DMPA, 5.0 mg, 0.038 mmol) was added. , nitrogen purged for 20min, under stirring conditi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com