Method for transforming dimethyl oxalate into ethylene glycol by one-pot process under hydrogen-free condition
A technology of dimethyl oxalate and ethylene glycol, which is applied in chemical instruments and methods, preparation of organic compounds, metal/metal oxide/metal hydroxide catalysts, etc. The activation of source hydrogen, the cumbersome preparation process, etc., can reduce the investment in equipment and hydrogen transportation, enhance the activity and stability, and enhance the ability to absorb and dissociate H.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
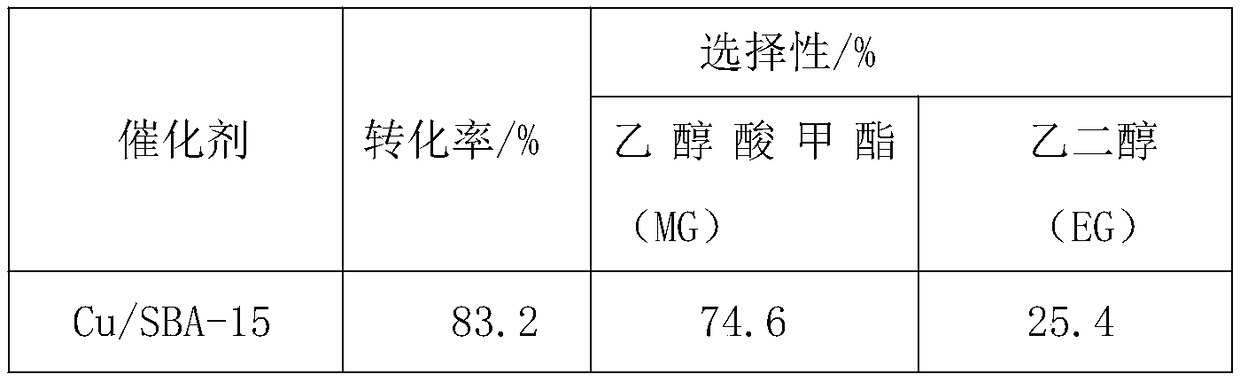
[0026] Application of Catalyst Cu / SBA-15 in Hydrogenation of Dimethyl Oxalate
[0027] (1) Catalyst prepared by hydrothermal method
[0028] Weigh 0.3382g copper nitrate trihydrate (Cu(NO 3 ) 2 ·3H 2 O), be dissolved in 140ml deionized water and be configured into a solution, take by weighing 1.498g ammonium chloride (NH 4 Cl) was added to the above solution and stirred to dissolve, and then the pH of the solution was adjusted to 10-12. Add 0.1764g of SBA-15 into the copper nitrate solution under stirring, stir at room temperature for 0.5h, and sonicate for 0.5h. Then the solution was transferred to a hydrothermal kettle and reacted dynamically in a homogeneous reactor at 120° C. for 3 h. After filtering and washing, the Cu-based catalyst precursor was obtained. The obtained Cu-based catalyst precursor was dried in an oven at 60 °C for 12 h, and finally calcined and reduced in a tube furnace at 450 °C for 4 h to obtain the catalyst Cu / SBA-15.
[0029] (2) Catalyst perfor...
Embodiment 2
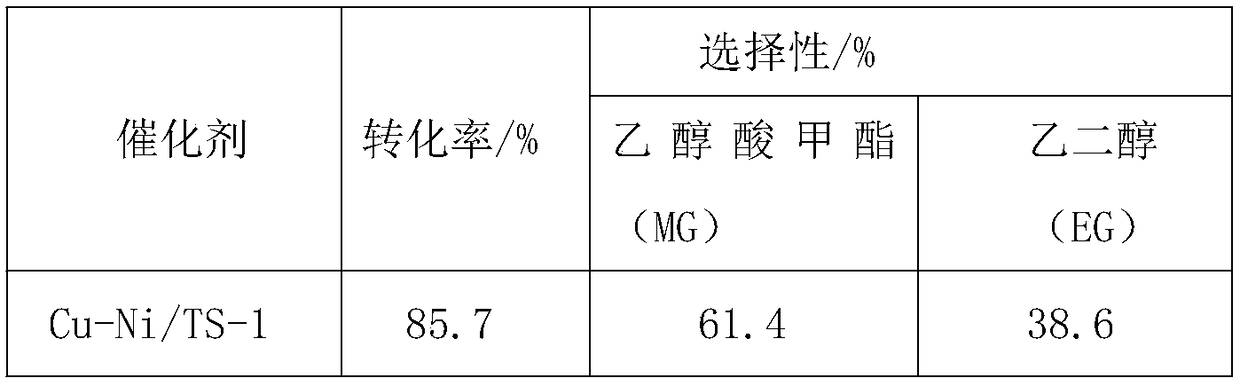
[0034] Application of Catalyst Cu-Ni / TS-1 in Hydrogenation of Dimethyl Oxalate
[0035] (1) Catalyst prepared by hydrothermal method
[0036] Take by weighing 0.3382g copper nitrate trihydrate (Cu(NO 3 ) 2 ·3H 2 O) and 0.1071g nickel nitrate hexahydrate (Ni(NO 3 ) 2 ·6H 2 O), dissolved in 140ml deionized water successively to form a solution, weighed 1.498g ammonium chloride (NH 4 Cl) was added to the above solution and stirred to dissolve, and ammonia water was added to adjust the pH of the solution to 10-12. Add 0.1764g TS-1 into the copper nitrate solution under stirring, stir at room temperature for 0.5h, ultrasonic for 0.5h, then transfer the solution to a hydrothermal kettle, and react dynamically in a homogeneous reactor at 120°C for 3h . After filtering and washing, the Cu-based catalyst precursor was obtained. The obtained Cu-based catalyst precursor was dried in an oven at 60°C for 12 hours, and finally calcined and reduced in a tube furnace at 450°C for 4 ho...
Embodiment 3
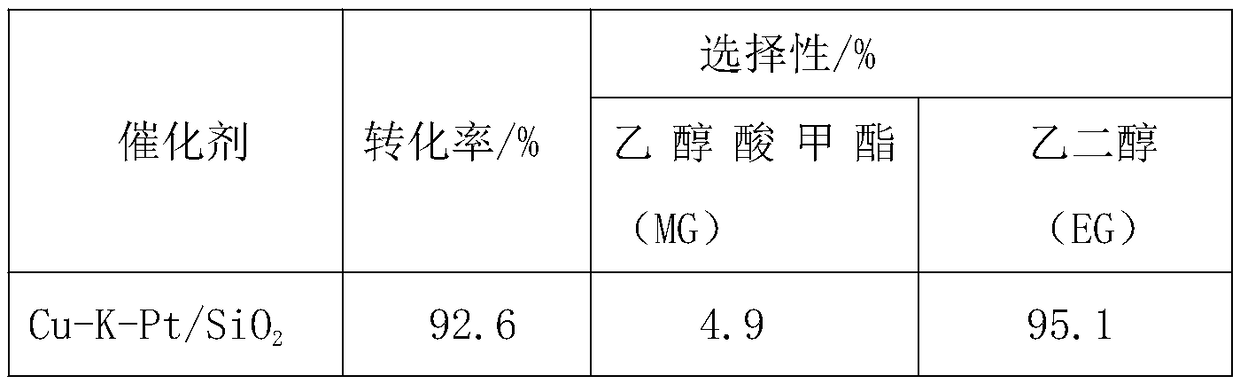
[0042] Catalyst Cu-K-Pt / SiO 2 Applied to the hydrogenation reaction of dimethyl oxalate
[0043] (1) Catalyst prepared by hydrothermal method
[0044] Take by weighing 0.3382g copper nitrate trihydrate (Cu(NO 3 ) 2 ·3H 2 O), 0.0169g potassium chloride (KCl), 0.0061g platinum tetrachloride (PtCl 4 ) was dissolved in 140ml deionized water to form a solution, and a mixed solution of ammonia / ammonium chloride was added to adjust the pH of the solution to 10-12, and 0.1764g SiO 2 Add it into the copper nitrate solution, stir at room temperature for 0.5h, and sonicate for 0.5h, then transfer the solution to a hydrothermal kettle, and react dynamically in a homogeneous reactor at 120°C for 3h. After filtering and washing, the Cu-based catalyst precursor was obtained, and the obtained Cu-based catalyst precursor was dried in an oven at 60°C for 12h, and finally calcined and reduced in a tube furnace at 450°C for 4h to obtain the catalyst Cu-K-Pt / SiO 2 .
[0045] (2) Catalyst ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com