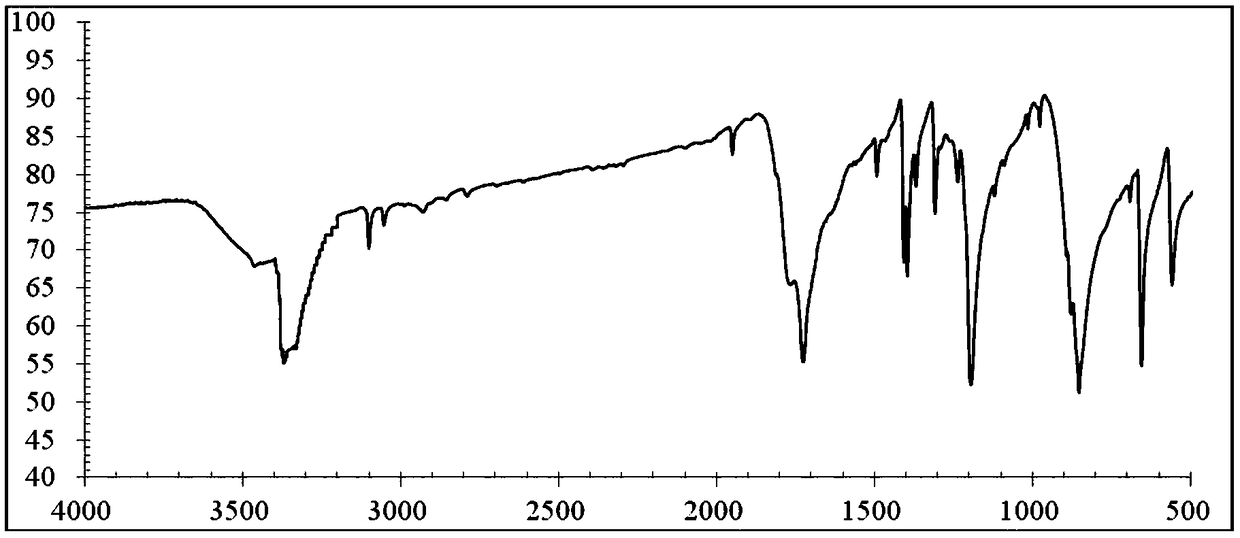
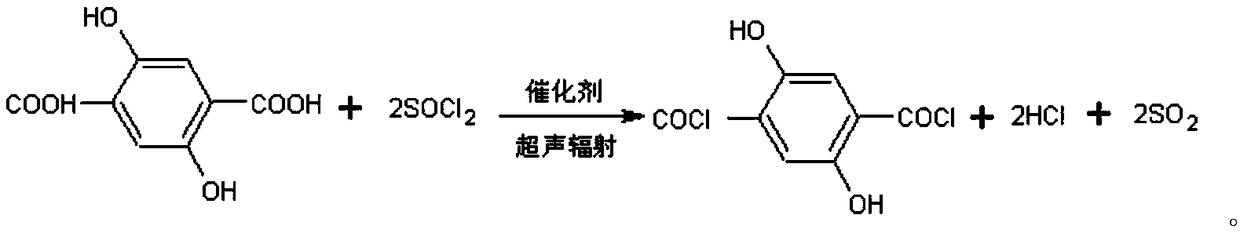
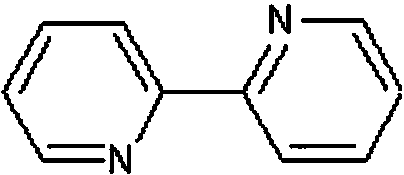
2,5-dyhydroxyl paraphthaloyl chloride and preparation method thereof
A technology of hydroxyterephthaloyl dichloride and hydroxyterephthalic acid, which is applied in the field of 2,5-dihydroxyterephthaloyl dichloride and its preparation, can solve the problems such as the decline of mechanical properties of products, the erosion of fiber surface, and the degradation of materials. , to achieve the effect of improving the bulk density and crystallinity, resisting erosion damage, and simple reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of preparation method of high-purity 2,5-dihydroxy terephthaloyl chloride of the present invention, comprises the steps:
[0036](1) Add 50g of 2,5-dihydroxyterephthalic acid, 61.1g of thionyl chloride and 0.4g of 2,2'-bipyridine into a round-bottomed flask with a reflux condenser, and put it into an ultrasonic instrument, Ultrasonic power 250W, ultrasonic reflux reaction at 80°C for 2h, the hydrogen chloride and sulfur dioxide generated by the reaction were passed into the potassium hydroxide solution with a mass concentration of 20% in time, and then the reaction was ended after no tail gas appeared, and the thionyl chloride and sulfur dioxide were distilled out under reduced pressure. Catalyst 2,2'-bipyridine to obtain 60.6 g of crude 2,5-dihydroxyterephthaloyl chloride, with an isolated yield of 90%;
[0037] (2) Add 0.31g FeCl to the crude 2,5-dihydroxyterephthaloyl chloride obtained after step (1) 3 Heated to 120°C, kept at constant temperature for 1 hour,...
Embodiment 2
[0042] A kind of preparation method of high-purity 2,5-dihydroxy terephthaloyl chloride of the present invention, comprises the steps:
[0043] (1) Add 50g of 2,5-dihydroxyterephthalic acid, 61.1g of thionyl chloride and 0.4g of 2,2'-bipyridine into a round-bottomed flask with a reflux condenser, and put it into an ultrasonic instrument, Ultrasonic power 250W, ultrasonic reflux reaction at 70°C for 2.5h, the hydrogen chloride and sulfur dioxide generated by the reaction were passed into the potassium hydroxide solution with a mass concentration of 20% in time, and then the reaction was ended after no tail gas appeared, and the thionyl chloride was distilled out under reduced pressure and catalyst 2,2'-bipyridine to obtain 58 g of crude 2,5-dihydroxyterephthaloyl chloride, with an isolation yield of 86%;
[0044] (2) Add 0.3g FeCl to the crude 2,5-dihydroxyterephthaloyl chloride obtained after step (1) 3 Heated to 120°C, kept at constant temperature for 1 hour, and vacuum dist...
Embodiment 3
[0048] A kind of preparation method of high-purity 2,5-dihydroxy terephthaloyl chloride of the present invention, comprises the steps:
[0049] (1) Add 50g of 2,5-dihydroxyterephthalic acid, 61.1g of thionyl chloride and 0.4g of 2,2'-bipyridine into a round-bottomed flask with a reflux condenser, and put it into an ultrasonic instrument, Ultrasonic power 250W, ultrasonic reflux reaction at 75°C for 3h, the hydrogen chloride and sulfur dioxide generated by the reaction were passed into the potassium hydroxide solution with a mass concentration of 20% in time, and then the reaction was ended after no tail gas was observed, and the thionyl chloride and sulfur dioxide were distilled out under reduced pressure. Catalyst 2,2'-bipyridine to obtain 60 g of crude 2,5-dihydroxyterephthaloyl chloride with an isolated yield of 89%;
[0050] (2) Add 0.3g FeCl to the crude 2,5-dihydroxyterephthaloyl chloride obtained after step (1) 3 Heated to 120°C, kept at constant temperature for 1 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com