Preparation method for polyvinylidene fluoride modified composite film
A technology of polyvinylidene fluoride and composite membrane, which is applied in chemical instruments and methods, membrane technology, semipermeable membrane separation, etc., can solve the problems of inconvenient catalyst recovery and poor catalytic degradation effect, and achieve good catalytic degradation effect and solve Effects of Recycling Questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A preparation method of a polyvinylidene fluoride modified composite membrane, comprising the steps of:
[0025] a) Graphene oxide (GO) was prepared by an improved Hummer's chemical method;
[0026] b) Preparation of aminated graphene oxide (NGO): performing amination modification on the graphene oxide obtained in step a) to obtain aminated graphene oxide;
[0027] c) Preparation of amino-functionalized graphene oxide / zinc oxide (NGO-ZnO) composite photocatalyst; dissolving the aminated graphene oxide obtained in step b) in absolute ethanol, ultrasonically dispersing, adding zinc acetate dihydrate, and heating to dihydrate After the zinc acetate is completely dissolved, add the ethanol solution of potassium hydroxide, then place it in the reaction kettle for reaction, wash and dry to obtain the NGO-ZnO composite photocatalyst;
[0028] d) Preparation of polyvinylidene fluoride modified composite membrane (PVDF / NGO-ZnO), as follows:
[0029] d1) After ultrasonically di...
Embodiment 1
[0054] a) Preparation of graphene oxide by modified Hummer's chemistry
[0055] 5g of pretreated graphite and 2.5g of sodium nitrate were added to a reactor containing 120ml of sulfuric acid, and after stirring for 1h under an ice-water bath, 15g of potassium permanganate was slowly added and stirring was continued for 2h; then the reaction system was heated to 35°C, After continuing to react for 2 hours, slowly add 120ml of deionized water, raise the temperature of the reaction system to 90°C and stir for 15 minutes; then slowly add 250ml of deionized water to the system, stir for 2 minutes and then add 12ml of 30wt% hydrogen peroxide to terminate the reaction, the color of the solution is From black to bright yellow, continue to stir for 15 minutes, pickle the product, wash with water to pH ≈ 7, filter and freeze-dry to obtain graphene oxide (GO), ready to use;
[0056] b) Preparation of aminated graphene oxide (NGO)
[0057] Disperse 30 mg of GO obtained in step a) in 50 m...
Embodiment 2
[0064] a)-c) with embodiment 1;
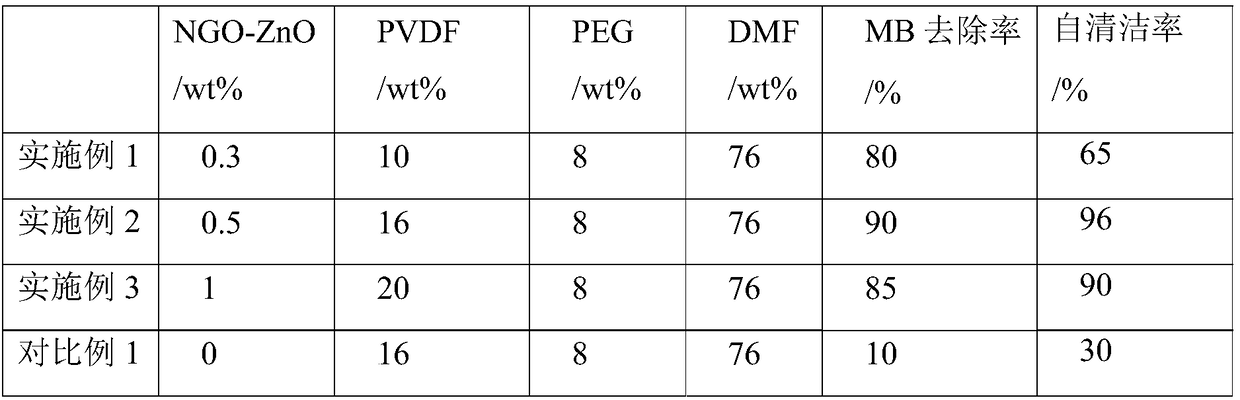
[0065] d) Preparation of polyvinylidene fluoride modified composite membrane
[0066] d1) 0.5 wt% of the NGO-ZnO photocatalyst prepared in step c) was ultrasonically dispersed in a DMF solution with a PEG concentration of 8 wt%, and 16 wt% of PVDF powder was added to the solution. The mixed solution was stirred at 60° C. until the PVDF was completely dissolved, and left at this temperature for defoaming for 12 hours to obtain a casting solution.
[0067] d2) Add the casting solution dropwise on a dry glass plate, and scrape a flat film with a film scraping rod with a thickness of 300 μm. Rapidly immerse the nascent film in a pure water coagulation bath at 25°C, wash it with a large amount of pure water after the film is formed, and store it in pure water to obtain a polyvinylidene fluoride modified composite film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com