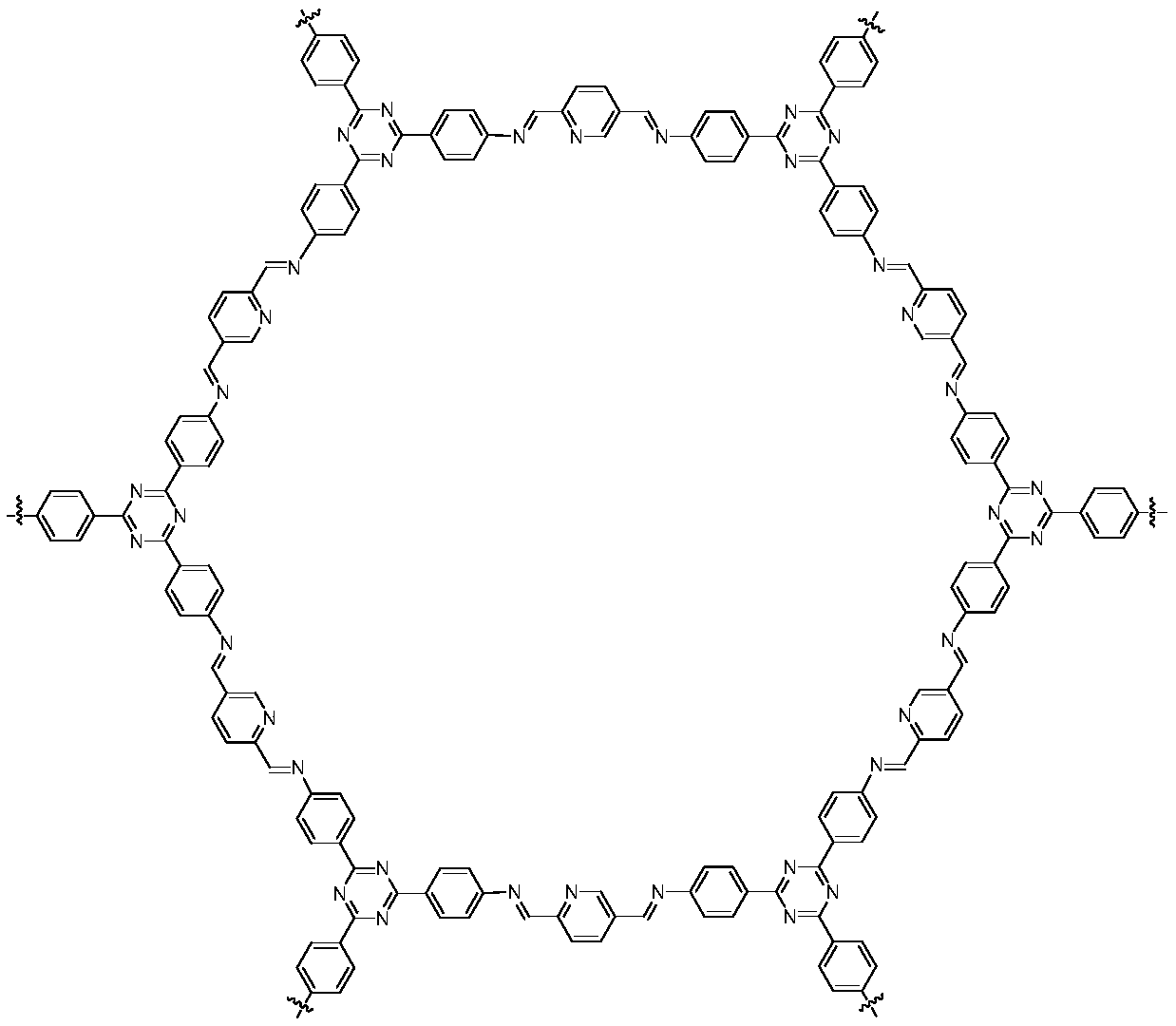
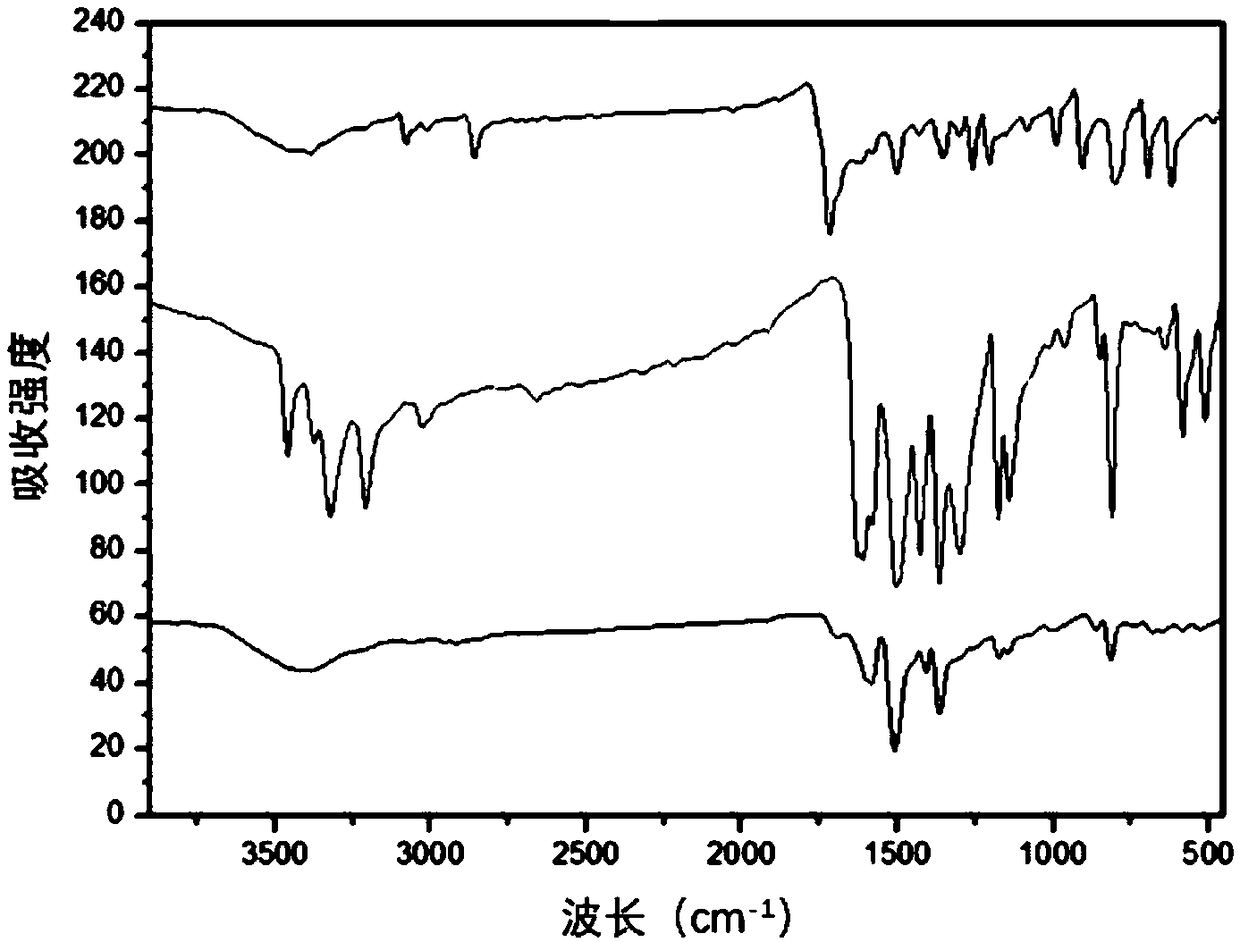
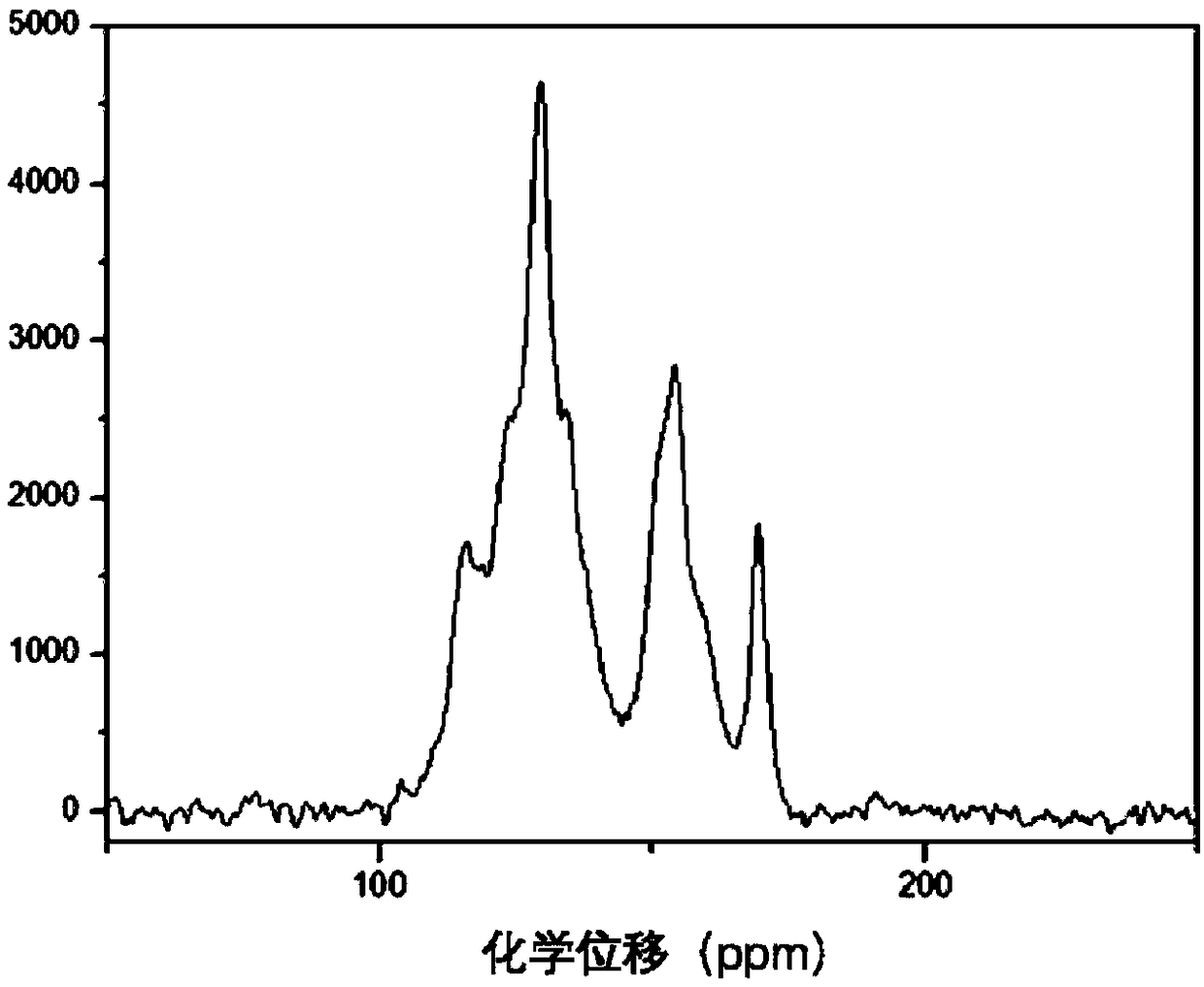
Covalent organic framework material, preparation method therefor and application of covalent organic framework material in synthesis of hindered amines
A covalent organic framework and organic solvent technology, applied in the field of synthesis and preparation of covalent organic framework materials, can solve problems such as low yield, decreased yield, and heavy metal pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0043] Preparation Example 1: Preparation of Catalyst C1
[0044] P1: Mix 2,5-pyridinedimethanol and selenium dioxide in the 1,4-dioxane solution at a molar ratio of 1.2:1, and react vigorously under reflux at 100°C. The reaction time is 7 hours. After completion, it was filtered and concentrated to obtain a solid, and the obtained solid was separated by column chromatography (developing solvent: dichloromethane: ethyl acetate = 2:1) to obtain a light pink solid, namely 2,5-diformylpyridine.
[0045] P2: The mass ratio of p-aminobenzonitrile and trifluoromethanesulfonic acid is 0.15:1, fully stirred and reacted at room temperature under nitrogen protection for 24 hours, then add appropriate amount of distilled water and adjust it to pH = 7 with 2M sodium hydroxide solution , filtered, washed several times with distilled water and dried to obtain a light yellow solid that is 2,4,6-tris(4-aminophenyl)-1,3,5-triazine.
[0046] P3: Mix the synthesized 2,5-diformylpyridine and 2,4...
preparation example 2
[0048] Preparation Example 2: Preparation of Catalyst C2
[0049] P1: Mix 2,5-pyridinedimethanol and selenium dioxide in a 1,4-dioxane solution at a molar ratio of 0.8:1, and react vigorously under reflux at 100°C. The reaction time is 4 hours. After completion, it was filtered and concentrated to obtain a solid, and the obtained solid was separated by column chromatography (developing solvent: dichloromethane: ethyl acetate = 2:1) to obtain a light pink solid, namely 2,5-diformylpyridine.
[0050] P2: The mass ratio of p-aminobenzonitrile and trifluoromethanesulfonic acid is 0.15:1, fully stirred and reacted at room temperature under nitrogen protection for 24 hours, then add appropriate amount of distilled water and adjust it to pH = 7 with 2M sodium hydroxide solution , filtered, washed several times with distilled water and dried to obtain a light yellow solid that is 2,4,6-tris(4-aminophenyl)-1,3,5-triazine.
[0051] P3: The synthesized 2,5-diformylpyridine and 2,4,6-tri...
preparation example 3
[0053] Preparation Example 3: Preparation of Catalyst C3
[0054] P1: Mix 2,5-pyridinedimethanol and selenium dioxide in a 1,4-dioxane solution at a molar ratio of 1:1, and react vigorously under reflux at 100°C. The reaction time is 6 hours. After completion, it was filtered and concentrated to obtain a solid, and the obtained solid was separated by column chromatography (developing solvent: dichloromethane: ethyl acetate = 2:1) to obtain a light pink solid, namely 2,5-diformylpyridine.
[0055] P2: The mass ratio of p-aminobenzonitrile and trifluoromethanesulfonic acid is 0.15:1, fully stirred and reacted at room temperature under nitrogen protection for 24 hours, then add appropriate amount of distilled water and adjust it to pH = 7 with 2M sodium hydroxide solution , filtered, washed several times with distilled water and dried to obtain a light yellow solid that is 2,4,6-tris(4-aminophenyl)-1,3,5-triazine.
[0056] P3: Mix the synthesized 2,5-diformylpyridine and 2,4,6-t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com