Method for detecting anti-human epidermal growth factor receptor 2 monoclonal antibody-MMAE conjugate neutralizing antibody in human serum
A technology of epidermal growth factor and monoclonal antibody, which is applied in the field of antibody-drug conjugate neutralizing antibody detection, can solve the problems of sensitivity discount and low sensitivity, and achieve the effects of eliminating interference, improving sensitivity, and improving neutralizing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
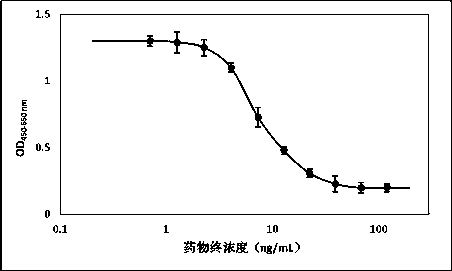
[0010] SK-BR-3 cell culture and proliferation inhibition experiment
[0011] Use culture flasks or culture dishes of appropriate specifications to culture SK-BR-3 cells (ATCC, HTB-30) TM ). at 75cm 2 Take the culture flask (Corning, 430641) as an example. The cell subculture process is as follows: Discard the medium, and wash the flask wall (including the cell layer) once with sterile phosphate buffered saline (hereinafter referred to as PBS). Add 1.5–2 mL of 0.25% Trypsin-EDTA (Gibco, 25200056) solution to the bottle and place at 37 °C, 5% CO 2 Digest for about 3 minutes in the incubator. Take out the culture bottle and observe the state of the cells under an inverted microscope. When the edge of the cells becomes oval and starts to fall off, pat the bottle wall to help the cells fall off, and then add 6-8 mL of complete medium to stop the digestion. Complete medium was prepared by mixing RPMI-1640 (Gibco, 31800022) medium with 10% FBS (Gibco, 10099141). Discard the exce...
Embodiment 2
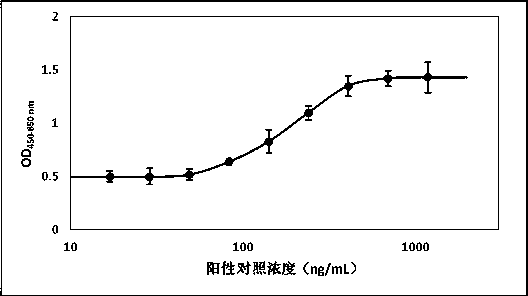
[0015] Preparation of anti-human epidermal growth factor receptor 2 monoclonal antibody-MMAE conjugate positive control antibody
[0016] Using cynomolgus monkeys, the anti-human epidermal growth factor receptor 2 monoclonal antibody-MMAE conjugate was used as the immunogen, referring to the classic preparation procedure of polyclonal antibody, and the positive control antibody was prepared by immunization method.
[0017] TiterMax® Gold Adjuvant (Sigma, T2684) was used as an immune adjuvant, mixed with the antigen to prepare an emulsion preparation, and multi-point subcutaneous injection was used to immunize cynomolgus monkeys (about 4-6kg), and the immune dose was 400μg. The anti-drug antibody titer in the serum was monitored 7 days after the initial immunization, and the F(ab)'2 fragment of the drug molecule was used as an antigen to boost the immunization 14 days later. Two weeks after the second immunization, venous blood was collected to evaluate the antibody titer. If t...
Embodiment 3
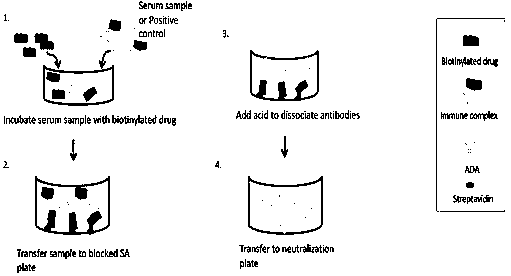
[0028] Extraction of ADA in Human Serum by Solid Phase Extraction
[0029] The composition of human serum samples is very complex, and drugs and other endogenous components may interfere with sample detection. For biological sample analysis methods, diluent dilution is usually used to reduce interference, and the minimum dilution factor (Minimum Required Dilution, MRD) that can eliminate the matrix effect is explored, but for cell culture-based neutralizing antibody methods, there is often little effect. Krista M. McCutcheon et al. adopted Protein A / G column chromatography to purify immunoglobulins in serum, so as to eliminate the influence of non-specific interfering substances in patient serum on the cell-based neutralizing antibody method (Journal of Immunological Methods, 358 , 2010), however, this method cannot eliminate blood drug interference. Solid phase extraction (SPE) is used to extract the ADA components in a directional manner, which can eliminate matrix interfer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com