Intravenous oncolytic virus preparation and preparation method thereof
A technology of oncolytic virus and limited dilution method, which is applied in the direction of resisting vector-borne diseases, medical preparations containing active ingredients, and medical raw materials derived from viruses/bacteriophages, etc., can solve problems such as obstacles to the development of cancer virus therapy, and achieve good results. Universal applicability, improved therapeutic effect, and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
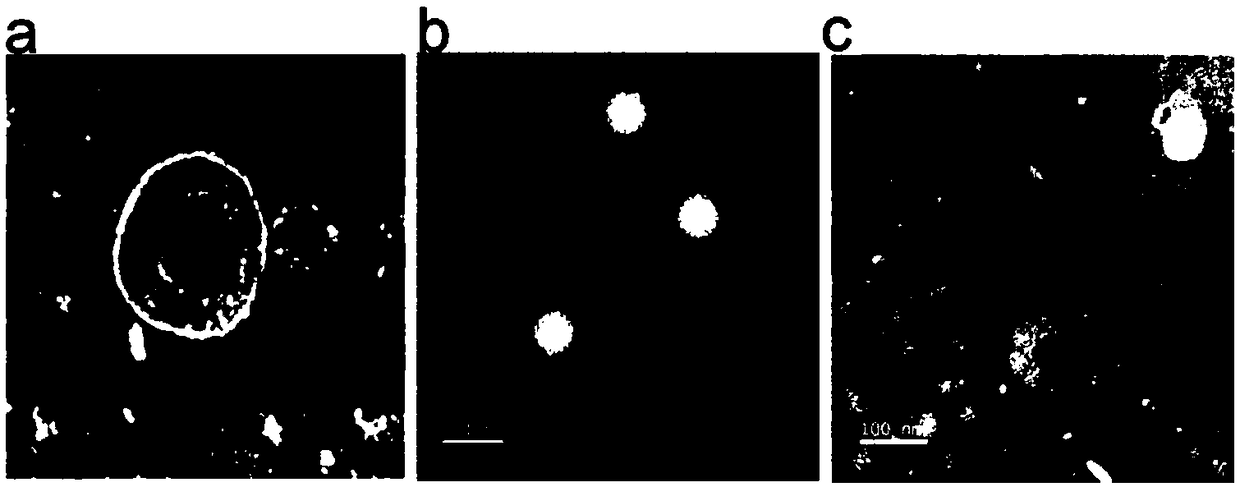
Image
Examples
preparation example
[0034] Preparation example: the reagents used in the following synthetic steps are commercially available.
[0035] A method for preparing an oncolytic adenovirus vaccine wrapped in cell membrane vesicles modified by targeting polypeptide hepatitis B virus capsid pre-S1 protein (preS1), comprising the following steps:
[0036] 1. Preparation of cell membrane vesicles modified by targeting polypeptide PreS1:
[0037] 1) 24 hours before transfection, HepG2 cells in the logarithmic growth phase were digested with trypsin, and the cell density was adjusted to 5×10 per ml with a medium containing 10% serum. 5 Cells were re-seeded in a 10cm cell culture dish at 37°C, 5% CO 2 Cultured in an incubator. When the cell density reaches 70%-80%, it can be used for transfection, and the cell culture medium is replaced with serum-free medium before transfection;
[0038] 2) Mix the diluted eukaryotic expression plasmid containing the PreS1 gene with the commercial transfection reagent Lip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com