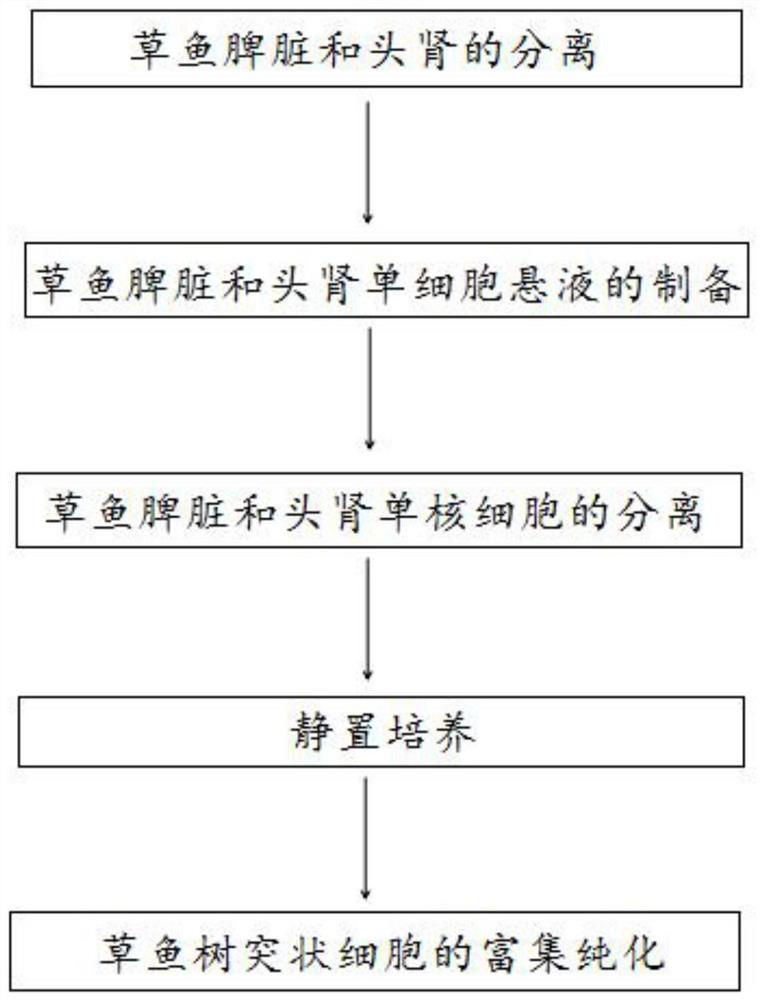
A method for the isolation and primary culture of grass carp dendritic cells
A dendritic cell and primary culture technology, which is applied in the field of grass carp dendritic cell isolation and primary culture, can solve problems such as the method of isolating and culturing grass carp dendritic cells that have not yet been seen, and reduce the risk of cell contamination Probability, less damage to the donor, and easy access to the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Preparation of experimental materials
[0035] The composition and proportion of the culture medium or culture medium used in the experiment are as follows
[0036] AIM solution: 47.50ml L-15 medium, 2.50ml double antibody, 2.5μg / mL amphotericin B and 25μg / mL gentamicin, put at 4℃ for later use;
[0037] Primary cell culture medium: 42.50ml L-15 medium, 5mL FBS, 2.50ml double antibody, 2.5μg / mL amphotericin B and 25μg / mL gentamicin, store at 4°C for later use;
[0038] 2. Experimental method
[0039] 1. Separation of spleen and head kidney of grass carp: anesthetize grass carp to death with excessive MS-222. The body surface was sprayed with 75% alcohol and repeatedly wiped and disinfected with alcohol cotton balls, then transferred to a sterile ultra-clean bench, and blood was collected from the tail vein.
[0040] Separation of the spleen: Use sterile surgical scissors to open the abdominal cavity along the lateral line of the fish body, and remove the spleen wi...
experiment example
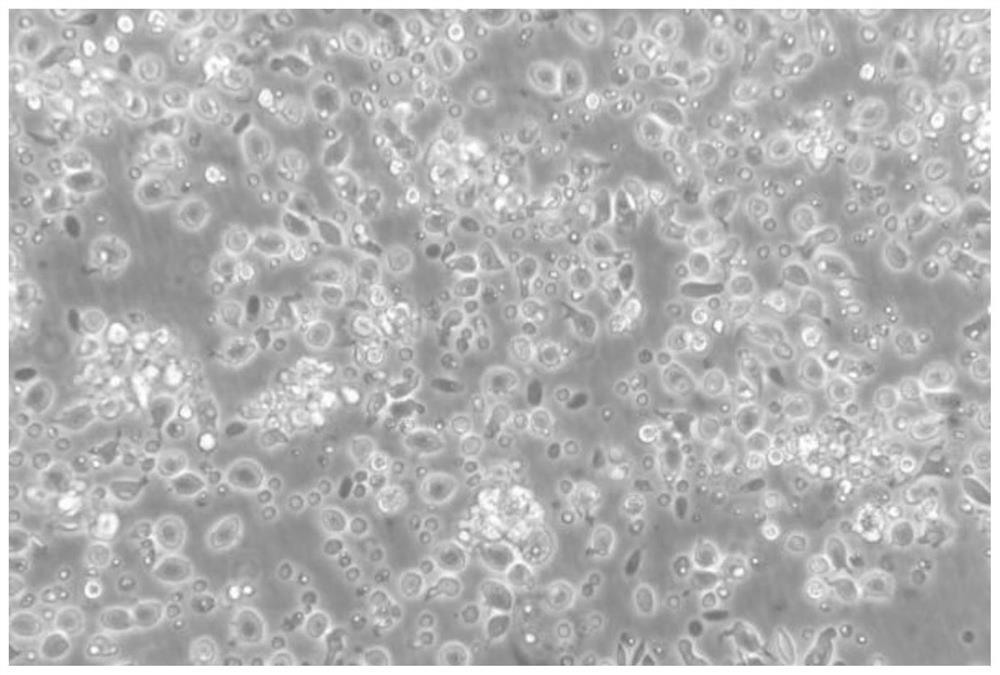
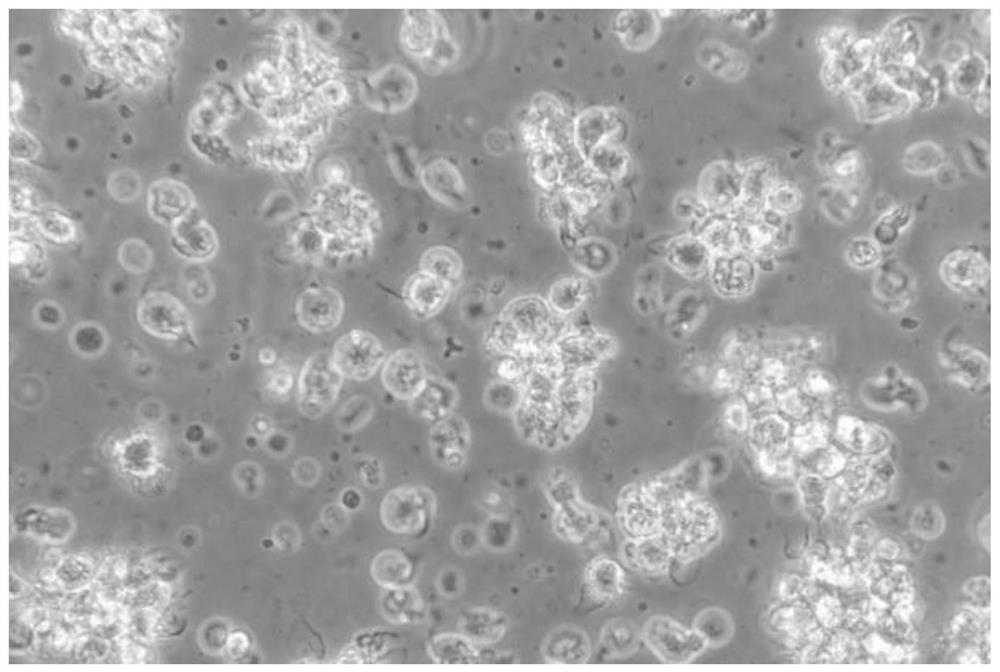
[0047] After the grass carp dendritic cells obtained in the above examples were enriched and purified, they were placed on a flow cytometer to detect the purity of the cells. In flow cytometry, forward scattered light (Forward scatter, FSC) reflects the size of the cell, side scattered light (Side scatter, SSC) reflects the complexity of the cell, the size and complexity of different types of cells The difference is large, so they can be divided into groups. Therefore, FSC and SSC of flow cytometry were used here to analyze the purity of dendritic cells after enrichment. Experimental results such as Figure 7 As shown, the whole cells are concentrated into a group, and there are very few cells outside the group, indicating that the obtained grass carp dendritic cells are of high purity, and the statistical function of the flow cytometer is used to calculate the purity of the grass carp dendritic cells as high as 80% %above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com