High enzyme activity aspartokinase mutant, engineering bacteria and preparation method of the mutant
A technology of aspartokinase and mutants, which is applied in the field of bioengineering and can solve problems such as heavy tasks, long cycles, and difficulty in further increasing the yield of target substances.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, the construction of recombinant escherichia coli strain
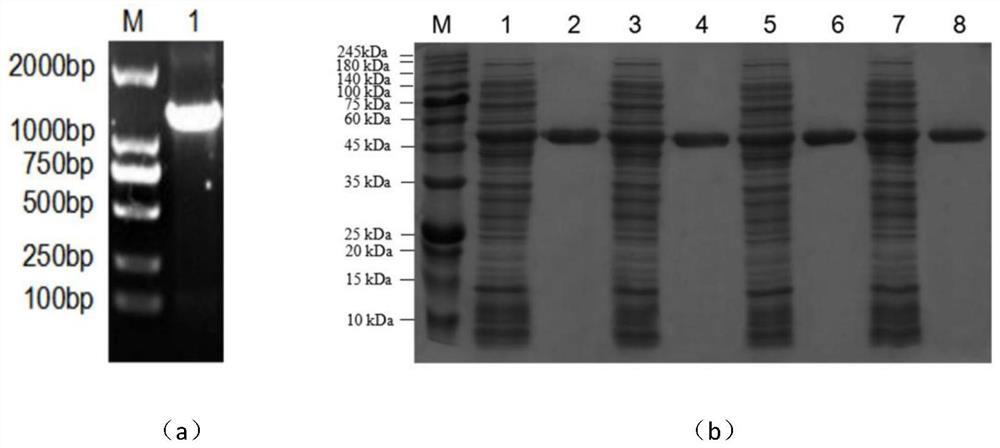
[0037] Using the TAKaRa Genome Extraction Kit to extract the chromosomal DNA of Corynebacterium pekinense, use it as a template to carry out PCR amplification under the action of cloning primers, clone a large number of AK target gene fragments, and verify by PCR nucleic acid electrophoresis, Corynebacterium pekinense Acid kinase (AK) in the figure 1 There are bright bands between 1000 and 2000 bp in the middle, which is consistent with the length of SEQ ID NO:1. All reagents were purchased from TAKaRa Company, and the designed cloning primers were as follows:
[0038] Upstream primer: 5'-GGAATTC CATATG GCCCTGGTCGTACAGAA-3'
[0039] Downstream primer: 5'-G GAATTC TTAGCGTCCGGTGCCTGCAT-3'
[0040] The underlined part is the restriction enzyme cutting site of NdeI and EcoRI restriction enzymes.
[0041]
[0042] (2) Construction of recombinant Escherichia co...
Embodiment 2
[0059] Embodiment 2, construction of aspartokinase mutant
[0060] (1) Construction of aspartokinase M372 site mutant
[0061] Using the pET-AK recombinant plasmid in Example 1 (2) as a template, primer 5'-GTAACACCTGGGTGAGACTG upstream of M372N NNN GCCCGCACC-3', downstream primer 5'-CTCGT GGGTGCGGGC NNN Under the action of CAGTCTCACCC-3' (the underlined part is the mutation site), the mutation PCR reaction is carried out, and the PCR product and the pET-28a vector are double-digested by restriction endonucleases EcoRI and NdeI and then ligated overnight (using Example 1 (2) in the double enzyme digestion and ligation system), the ligated product was transferred into Escherichia coli BL21 (DE3) competent by implementing the method of 1 (2), and aspartokinase M372 site mutant was obtained strain.
[0062]
[0063]
[0064] (2) Construction of aspartokinase T379 site mutant
[0065] Using the pET-AK recombinant plasmid in Example 1 (2) as a template, using the PCR react...
Embodiment 3
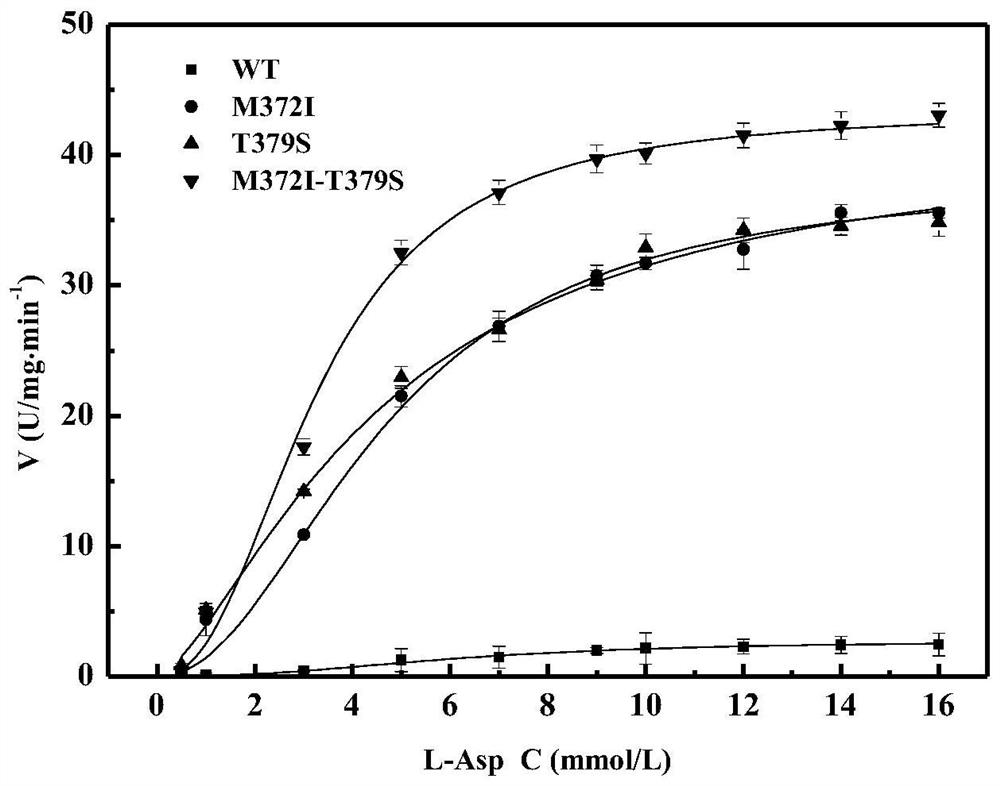
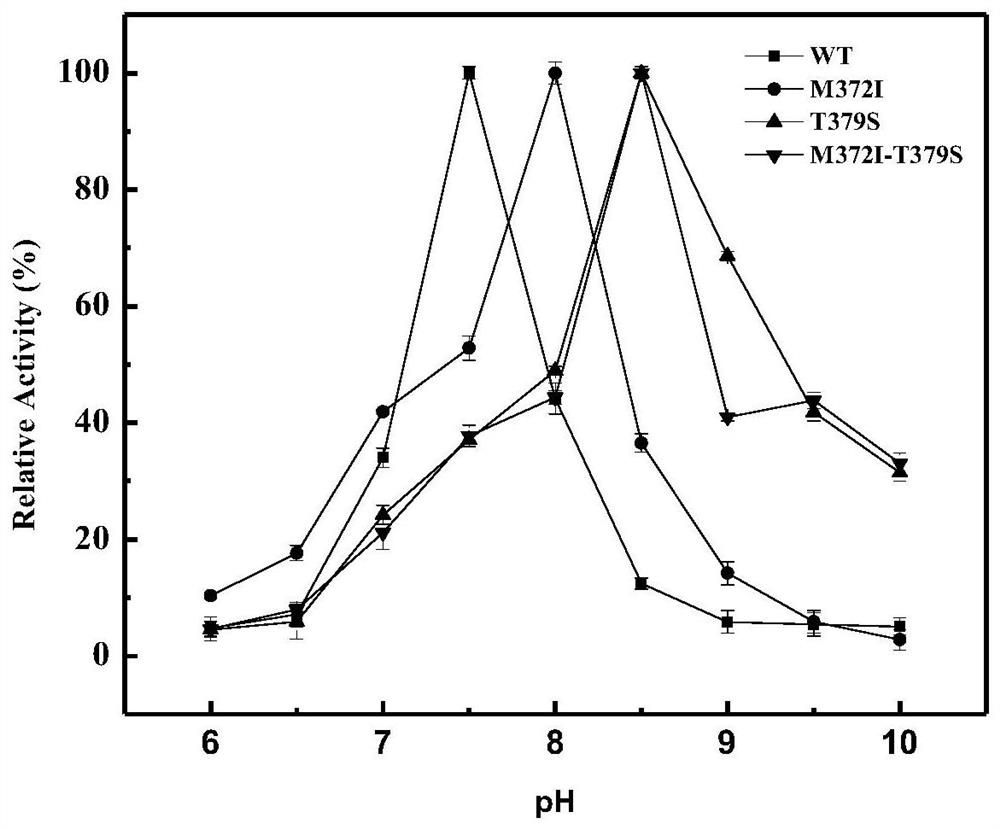
[0074] Embodiment 3, enzyme kinetic analysis and enzymatic property characterization
[0075] (1) Separation and purification of crude enzyme liquid
[0076] The recombinant escherichia coli strain (WT) of embodiment 1 (2) was inoculated with the high enzyme activity mutant (single mutant M372I and T379S) and double mutant M372I-T379S in embodiment 2 with 2% (v / v) The amount was transferred to 100mL LB liquid medium containing kanamycin, cultured overnight at 37°C, 180r / min, then added IPTG inducer (final concentration 1mmol / L), induced at 30°C, 130r / min for 12h . The bacterial solution was centrifuged at 8000r / min for 10min, the supernatant was discarded, and 10mL of PBS was added to resuspend the bacteria, and the supernatants obtained by ultrasonication for 30min and centrifugation at 8000r / min for 10min were respectively WT crude enzyme solution, M372I crude enzyme solution, and T379S crude enzyme solution. solution and M372I-T379S crude enzyme solution. The crude enzym...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com