Method for preparing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate through condensation of isobutylaldehyde
A technology of pentanediol monoisobutyrate and isobutyraldehyde, which is applied in aldehyde redox preparation, separation/purification of carboxylate, organic chemistry, etc., can solve the problem of high quality raw material isobutyraldehyde and complex reaction process , catalyst toxicity and other problems, to achieve the effect of environmentally friendly synthesis process, high yield, and less amount of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In this embodiment, a 100mL reaction kettle was used and heated in an oil bath. Add 50g of isobutyraldehyde to the reaction kettle, then add 0.5g of 1-methyl-3-butyl-imidazolium tetrafluoroborate, seal the reaction kettle, heat to 100°C, react for 20h, cool down after the reaction is completed , the measured yield of alcohol ester-12 was 71.8%, and the total impurity content was 1.56%.
Embodiment 2
[0033] Use a 1000L reactor and use steam heating. Add 500Kg isobutyraldehyde in the reactor, add 10Kg tetrabutylammonium hydroxide again, seal the reactor, heat to 80 ℃, react for 24h, after the reaction is completed, the reaction material is cooled down, and the yield of alcohol ester-12 is measured as 75.6%, and the total impurity content is 1.28%.
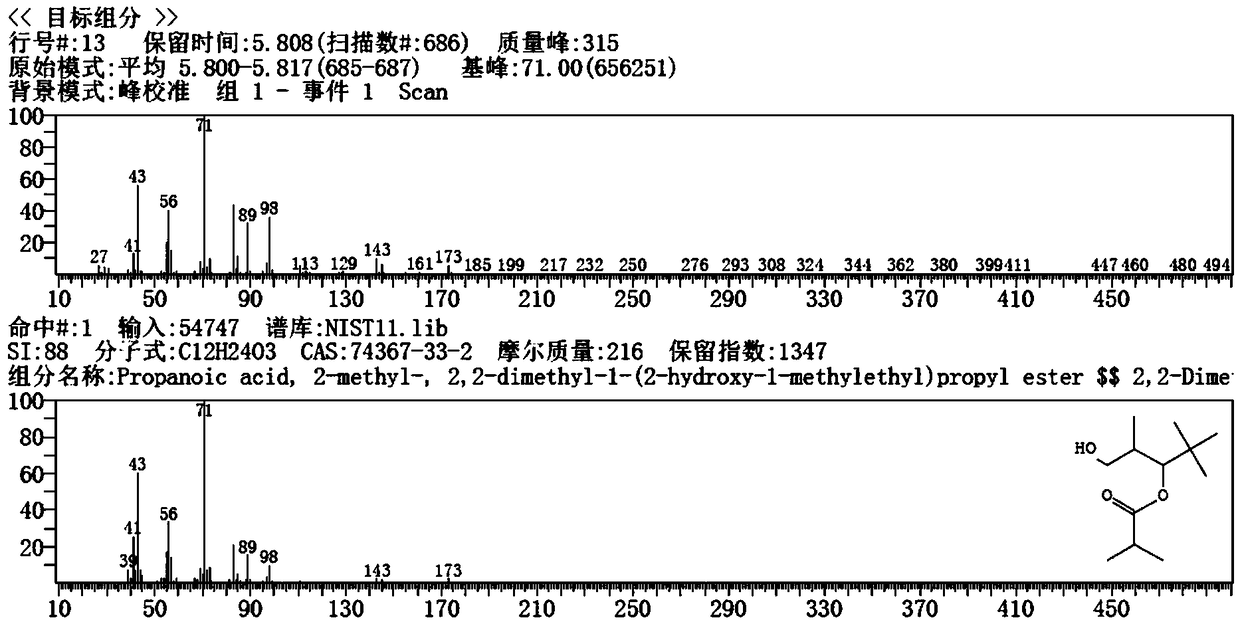
[0034] The reaction materials are sent to three rectification towers in series for rectification, and alcohol ester-12 is extracted from the top of the third rectification tower. The mass spectrometry test results are shown in figure 1 As shown in Figure A, with figure 1 Figure B is completely consistent with a purity of 99.5%. The ionic liquid catalyst remaining in the tower kettle is recovered.
Embodiment 3
[0036] In this embodiment, a 100mL reaction kettle was used and heated in an oil bath. Add 50g isobutyraldehyde in reactor, and add the ionic liquid catalyst (tetrabutylammonium hydroxide) that 0.25g embodiment 2 reclaims, be heated to 200 ℃, react 20h, measure the yield of alcohol ester-12 after completion of reaction is 73.6%, and the total impurity content is 1.18%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com