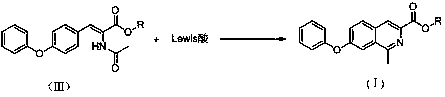Roxadustat key intermediate and synthetic method thereof
A technology of roxadustat and its synthetic method, which is applied in the field of organic synthesis route design and the synthesis of raw materials and intermediates, can solve the problems of unsuitability for industrial production, harsh reaction conditions, and long reaction steps, and achieve low equipment requirements, Mild reaction conditions and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
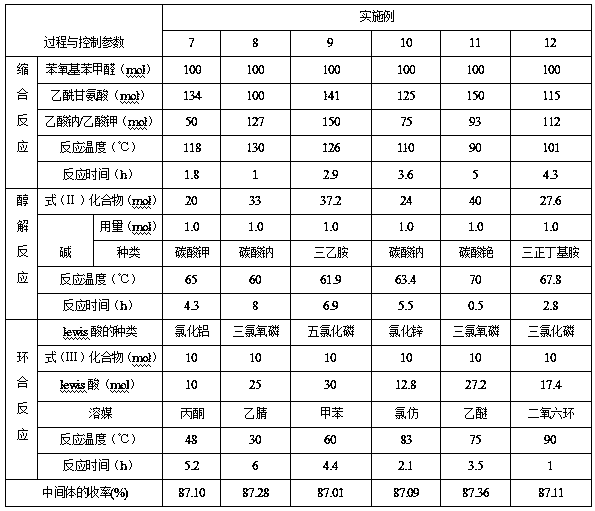
Embodiment 1~5
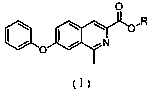
[0056] Embodiment 1~5 key intermediate of roxadustat
[0057] Examples 1-5 are respectively a key intermediate of roxadustat, methyl-7-phenoxyisoquinoline-3-carboxylate, which have the structure of formula (I),
[0058]
[0059] In the formula (I), the R group is an alkyl group containing 1 to 5 carbon atoms, and the R groups in Examples 1 to 5 are respectively methyl, ethyl, isopropyl, n-butyl or tert-butyl, See the table below for details:
[0060]
Embodiment 2
[0065] The methyl-7-phenoxyisoquinoline-3-carboxylic acid ethyl ester of embodiment 2
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com