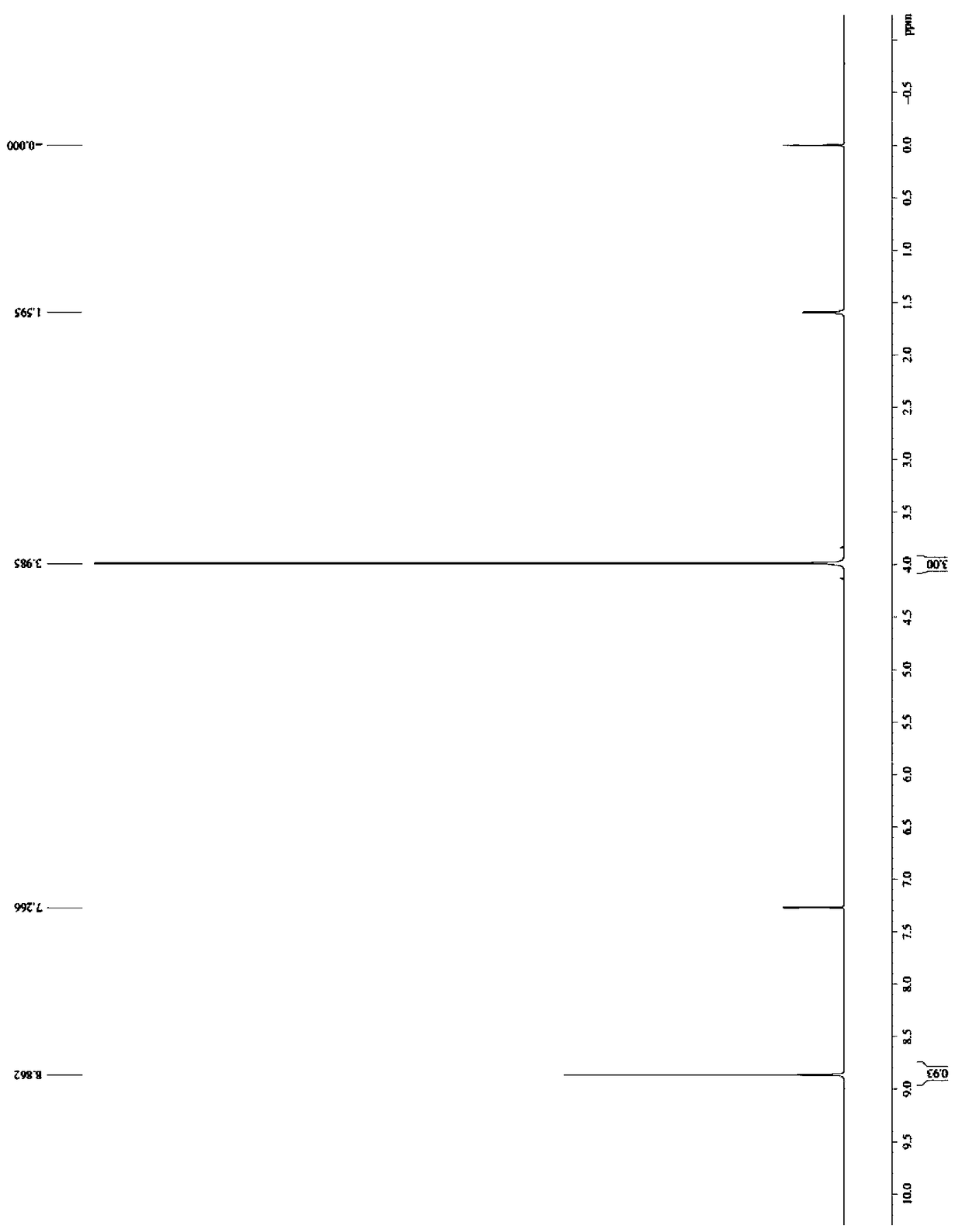
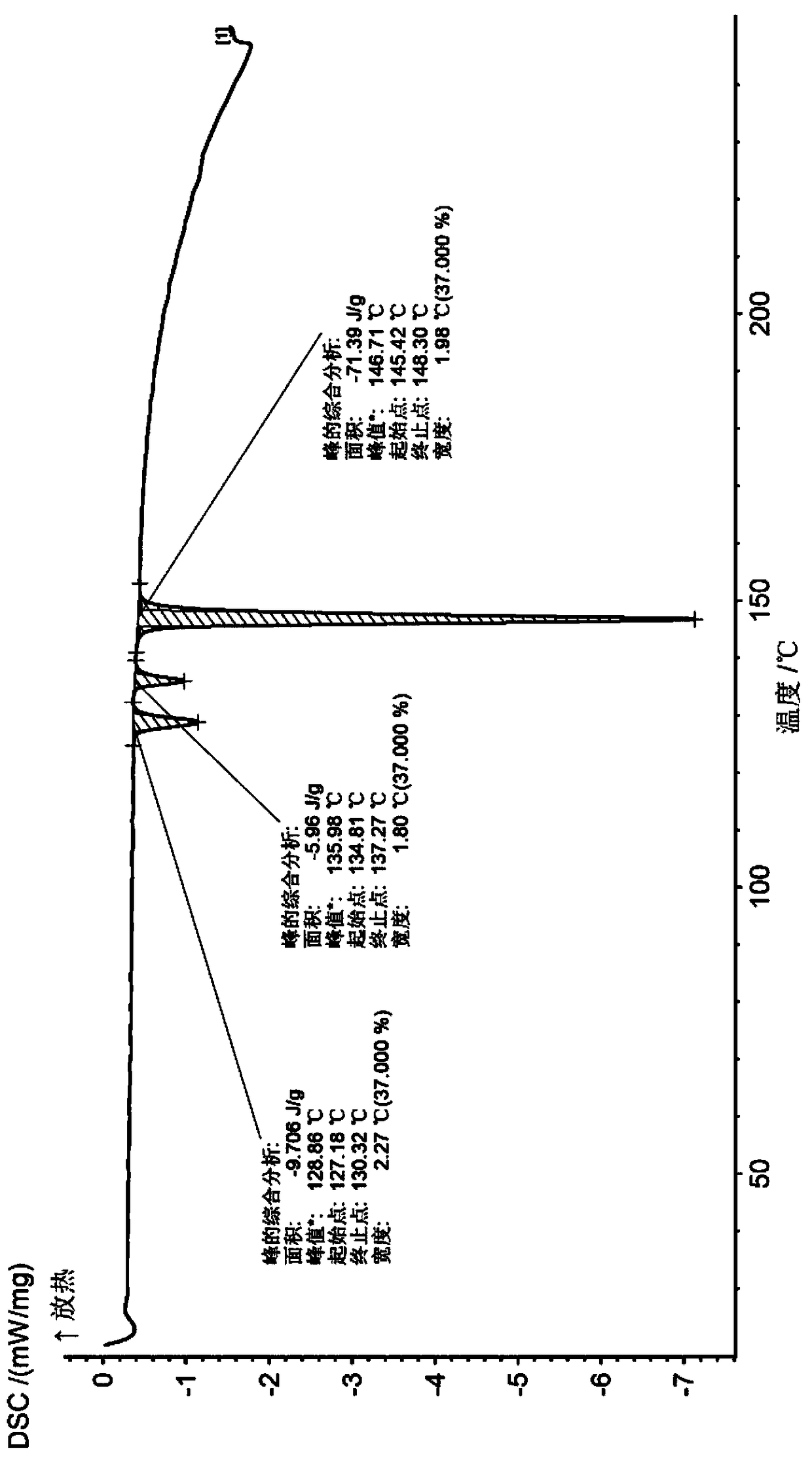
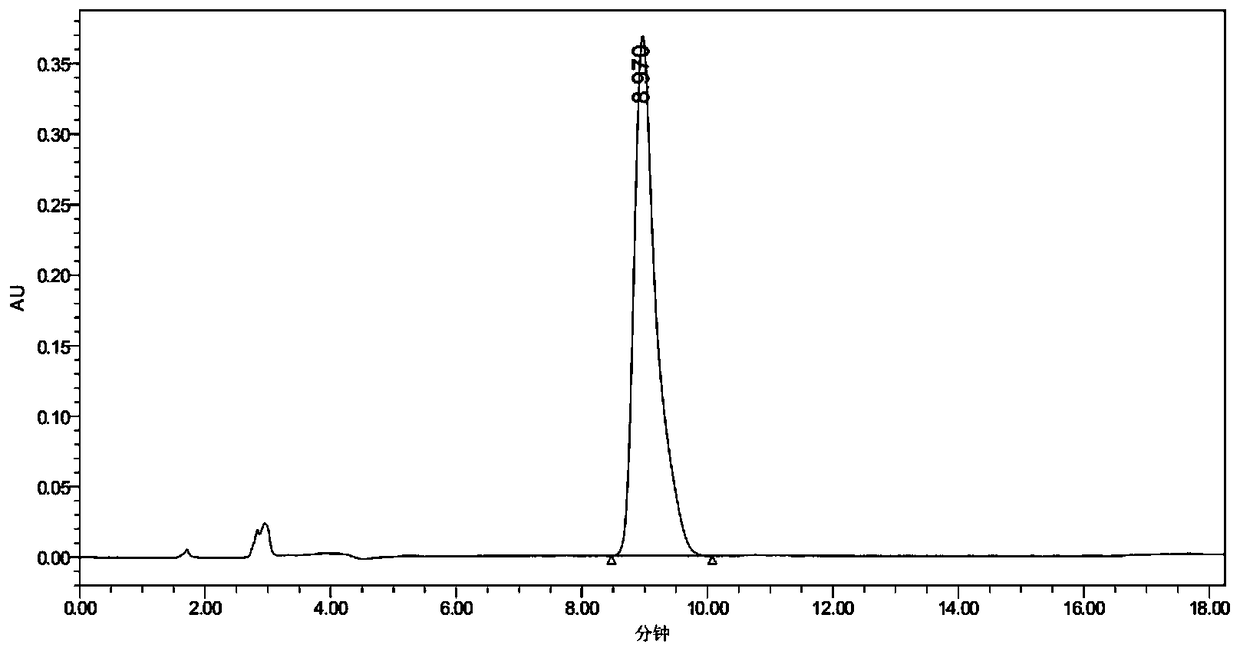
Synthesis method and application for trimethyl 1,3,5-benzene tricarboxylate
A technology of trimethylbenzenetricarboxylate and trimethyltricarboxylate, which is applied in the field of pharmacy, can solve the problems of long reaction time, failure to represent the separation yield, and the separation method provided by the reactant, so as to achieve the simple separation method and the operation process easily controlled effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 A kind of synthetic method of 1,3,5-trimethylbenzenetricarboxylate
[0043] (1) Self-molecular condensation reaction
[0044] Add 4.3kg of sodium methoxide (base, as a catalyst) to 50L of solvent N,N-dimethylformamide (DMF) at 0°C, stir for 0.5h to disperse into a suspension, heat up to 25°C and add 10kg 3-methoxy methyl acrylate, after stirring for 0.5h, generate the intermediate state of 6-methoxy-1,3,5-hexatriene-1,3,5-tricarboxylic acid trimethyl ester;
[0045] (2) Cyclization reaction
[0046] Slowly raise the temperature to 35°C, and control the temperature at 35°C to continue the reaction for 3 hours. The intermediate state obtained in step (1) produces a reaction solution A. Add the reaction solution A to 200L of ice water, and adjust the pH to 6.52 with hydrochloric acid (adjust with a pH meter). Stirring was continued for 1 h until white flocculent solids were completely precipitated. Filter and dry to obtain 1,3,5-trimethyl benzenetricarboxyl...
Embodiment 2
[0047] Embodiment 2 A kind of synthetic method of 1,3,5-trimethylbenzenetricarboxylate
[0048] (1) Self-molecular condensation reaction
[0049] Add 4.3kg of sodium methoxide (base, as a catalyst) to 50L of solvent N,N-dimethylformamide (DMF) at 0°C, stir for 0.5h to disperse into a suspension, heat up to 25°C and add 10kg of methyl 3,3-dimethoxypropionate, stirred for 0.5h to generate the intermediate state of trimethyl 6-methoxy-1,3,5-hexatriene-1,3,5-tricarboxylate ;
[0050] (2) Cyclization reaction
[0051] Slowly raise the temperature to 35°C, and control the temperature at 35°C to continue the reaction for 3 hours. The intermediate state obtained in step (1) produces a reaction solution A. Add the reaction solution A to 200L of ice water, and adjust the pH to 6.52 with hydrochloric acid (adjust with a pH meter). Stirring was continued for 1 h until white flocculent solids were completely precipitated. Filter and dry to obtain 1,3,5-trimethyl benzenetricarboxylate. F...
Embodiment 3~8
[0052] Embodiment 3~8 1,3, the synthetic method of 5-benzene trimethyl carboxylate
[0053] Examples 3 to 8 are respectively a synthesis method of 1,3,5-trimethylbenzenetricarboxylate. The operation steps are the same as those in Example 1 and Example 2, except for the types and amounts of the reaction raw materials, and The reaction time, reaction temperature, pH value and other control parameters in the process are different, see the following table for details:
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com