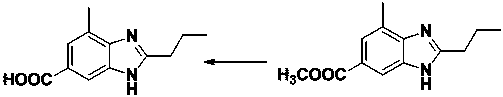Preparation method of telmisartan intermediate
A technology for telmisartan and intermediates, applied in the field of preparation of telmisartan intermediate 2-n-propyl-4-methyl-6-carboxybenzimidazole, which can solve the problem that the process route is not easy to control, color Not easy to remove, unstable product quality and other problems, to achieve the effect of improving production safety, avoiding carboxyl esterification, and reducing alkali hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
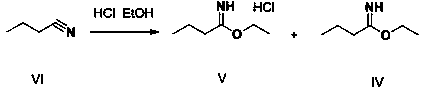
[0047] Step 1. Add 150 g of hydrochloric acid ethanol and 250 g of butyronitrile to the reaction flask R1, control the temperature at 20°C and maintain it, slowly inject 125 g of anhydrous hydrogen chloride gas, after the ventilation is complete, stir for 2 hours. 550 g of xylene was dropped into R1 to obtain a butyronitrile salt solution containing intermediates V and IV.
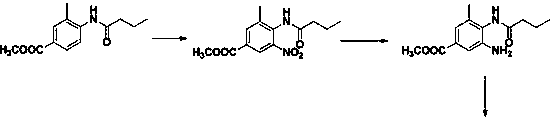
[0048] Step 2. Put 64g of sodium carbonate and 370g of water into the reaction bottle R2, stir until it dissolves, add 1500g of xylene, control the temperature at 10°C and maintain it, and start double-dropping the sodium hydroxide solution and the butane obtained in step 1 under stirring. Nitrile salt solution, maintain the pH value at 6.1, after the dropwise addition, stir for 2h. Separate the lower aqueous phase, and transfer the material to the R3 reaction flask. Under stirring, add 360 g of anhydrous methanol, 200 g of aminobenzoic acid, and 80 g of glacial acetic acid into the reaction flask R3, con...
Embodiment 2
[0051] Step 1. Add 75 g of ethanol hydrochloride and 250 g of butyronitrile to the reaction flask R1, control the temperature at 0°C and maintain it, slowly feed 163 g of anhydrous hydrogen chloride gas, complete the ventilation, and stir the reaction for 1.5 h. Put 500 g of xylene into R1 to obtain a butyronitrile salt solution containing intermediates V and IV.
[0052] Step 2. Put 62.5g of sodium carbonate and 370g of water into the reaction flask R2, stir until dissolved, add 1700g of xylene, control the temperature at 0°C and maintain it, and start double-dropping sodium hydroxide solution and the obtained product in step 1 under stirring Butyronitrile salt solution, maintain the pH value at 11.0, after the dropwise addition, stir for 1.5h. Separate the lower aqueous phase, and transfer the material to the R3 reaction flask. Under stirring, add 300 g of anhydrous methanol, 198 g of aminobenzoic acid, and 86 g of glacial acetic acid into the reaction flask R3, control the...
Embodiment 3
[0055] Step 1. Add 200 g of hydrochloric acid ethanol and 250 g of butyronitrile to the reaction flask R1, control the temperature at 35°C and maintain it, slowly feed 117.5 g of anhydrous hydrogen chloride gas, complete the ventilation, and stir for 1.5 hours. Put 500 g of xylene into R1 to obtain a butyronitrile salt solution containing intermediates V and IV.
[0056] Step 2. Put 67.6g of sodium carbonate and 370g of water into the reaction bottle R2, stir until it dissolves, add 1400g of xylene, control the temperature at 20°C and maintain it, and start double-dropping sodium hydroxide solution and the result obtained in step 1 under stirring Butyronitrile salt solution, maintain the pH value at 5.0, after the dropwise addition, stir for 1.5h. Separate the lower aqueous phase, and transfer the material to the R3 reaction flask. Under stirring, add 370 g of anhydrous methanol, 232 g of aminobenzoic acid, and 76 g of glacial acetic acid into the reaction bottle R3, control ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com