Anti-sea snake neurotoxin SN160 nano antibody, preparation method and application
A nano-antibody and neurotoxin technology, applied in the preparation of sea snake anti-venom preparations, treatment or prevention of sea snake bites, can solve the problems of strong patient, unstable quality, and difficulty in catching sea snakes, and achieve simple construction and expression process and excellent prevention Or therapeutic effect, the effect of broad clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Construction of Nanobody Library against Sea Snake Neurotoxin SN160
[0041] (1) 0.5 mg sea snake neurotoxin SN160 (Hu Shi et al., Screening, preparation and biological activity research of fully human monoclonal antibody against short-chain neurotoxin from flat-chin sea snake. PLA Medical Journal 42.7(2017):612-616.) The antigen was mixed with Freund's adjuvant in equal volumes, and a Xinjiang Bactrian camel was immunized once a week for a total of 6 consecutive immunizations. During the immunization process, B cells were stimulated to express specific nanobodies;
[0042] (2) After the 6 immunizations, extract 200 mL of camel peripheral blood lymphocytes and extract total RNA;
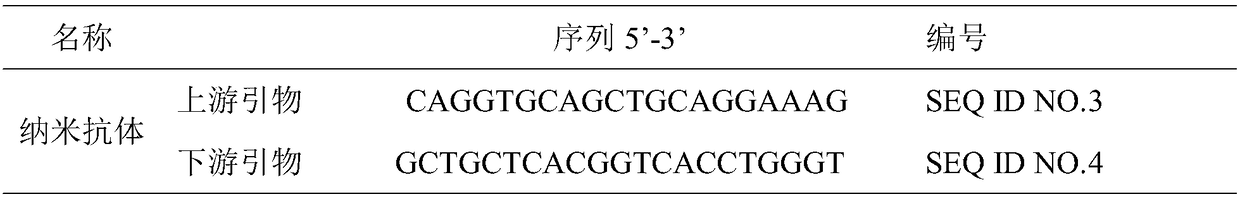
[0043] (3) Synthesize cDNA and utilize nested PCR to amplify VHH. The primer sequences used in this step are shown in Table 1:
[0044] Table 1 PCR primer sequences
[0045]
[0046] (4) Digest 20 μg of pMECS phage display vector and 10 μg of VHH with restriction enzymes Pstl a...
Embodiment 2
[0048] Example 2. Nanobody screening process against sea snake neurotoxin SN160
[0049] (1) 200 μL of recombinant TG1 cells were cultured in 2TY medium, during which 50 μL of helper phage VCSM13 was added to infect TG1 cells, and cultivated overnight to amplify the phages, and the next day, the phages were precipitated with PEG / NaCl, and the amplified phages were collected by centrifugation;
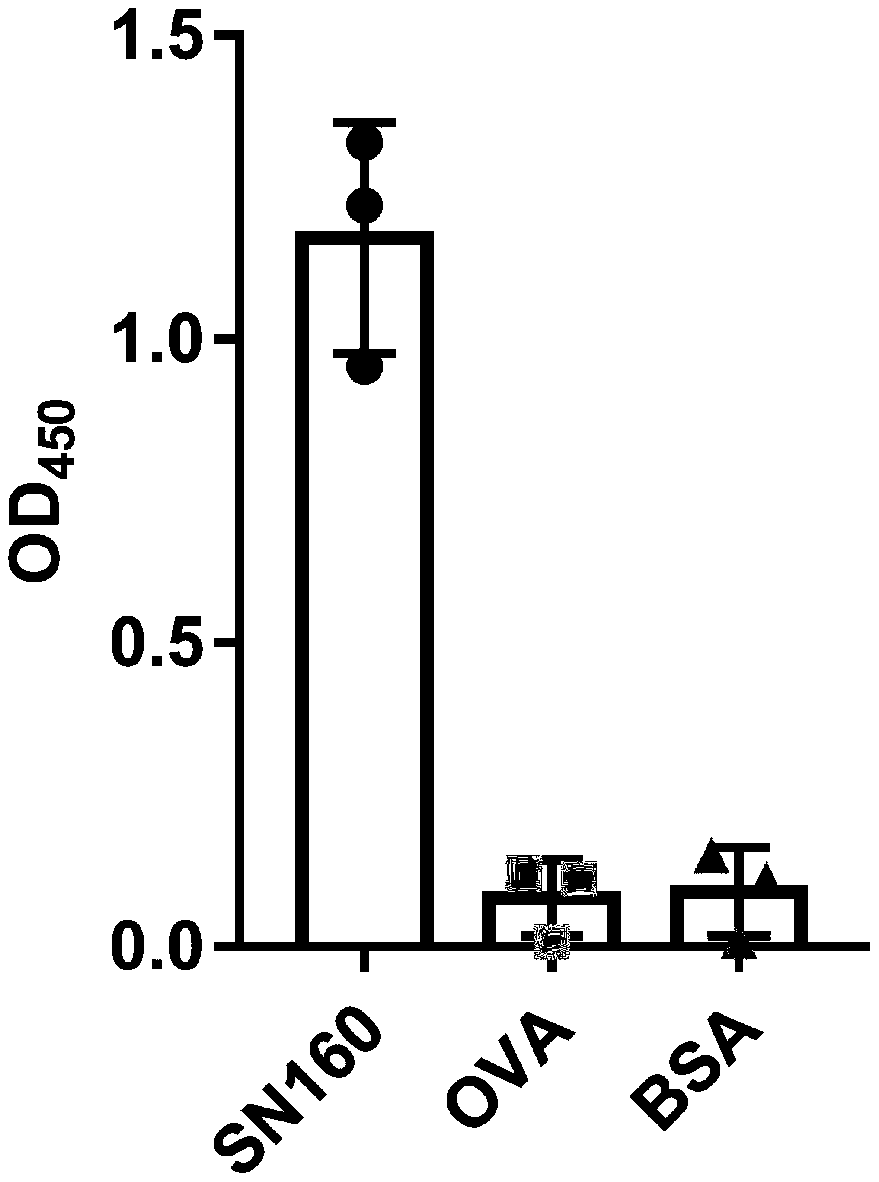
[0050] (2) Dissolve in 150mmol / L pH 8.2NaHCO 3 The sea snake neurotoxin SN160 in 150 μg was coupled to a microtiter plate, placed overnight at 4°C, and a negative control was set up at the same time;
[0051] (3) Add 100 μL of 5% BSA the next day, and block for 2 hours at room temperature;
[0052] (4) After 2 hours, add 100 μL of amplified phage (1×10 11 tfu immunized camel nanobody phage display gene library) at room temperature for 1 hour;
[0053] (5) Wash five times with PBS+0.05% Tween 20 to wash off bound phage;
[0054] (6) Use trypsin at a final concentration of 25 mg / mL to...
Embodiment 3
[0055] Example 3. Screening specific positive clones with phage enzyme-linked immunoassay (ELISA)
[0056] (1) Select 200 single colonies from the cell culture plates after the above three rounds of screening and inoculate them into 96 deep-well plates containing 100 μg / mL ampicillin TB medium, and set up a blank control, and culture at 37°C until the logarithmic phase After that, add IPTG with a final concentration of 1 mmol / L, and cultivate overnight at 28°C;
[0057] (2) Use the osmotic bursting method to obtain the crudely extracted antibody, and transfer the antibody to an antigen-coated ELISA plate, and place it at room temperature for 1 hour;
[0058] (3) Unbound antibodies were washed away with PBST, and 100 μL of Mouse anti-HAtagantibody (mouse anti-HA antibody, purchased from Covance) diluted 1:2000 was added, and left at room temperature for 1 hour;
[0059] (4) Unbound antibodies were washed away with PBST, 100 μL of Anti-mousealkalinephosphate conjugate (goat ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com