Freeze-dried (R)-lansoprazole powder injection, and preparation method and application thereof
A technology of dexlansoprazole and freeze-dried powder injection, which is applied in the field of medicine and can solve problems such as bleeding, dysphagia, and inappropriate oral medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 freeze-dried powder injection
[0033] Prepare respectively 10mg, 20mg, 30mg, 60mg and 90mg dexlansoprazole freeze-dried powder injection according to the prescription in Table 3.
[0034] Table 3 prescription composition
[0035]
[0036] The preparation process is as follows:
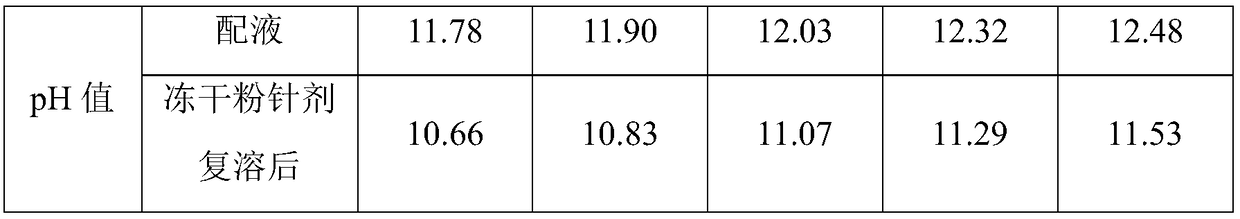
[0037] (1) Dosing: add mannitol, meglumine, dexlansoprazole and sodium hydroxide in the prescribed amount to the water for injection, stir until completely dissolved, and adjust the pH to between 11.8-12.3;
[0038] (2) Filtration: add 0.1% activated carbon for needles, stir at room temperature for 15min-30min, coarsely filter out carbon with a titanium rod, filter with a 0.45μm filter membrane, and finely filter with a 0.22μm filter membrane.
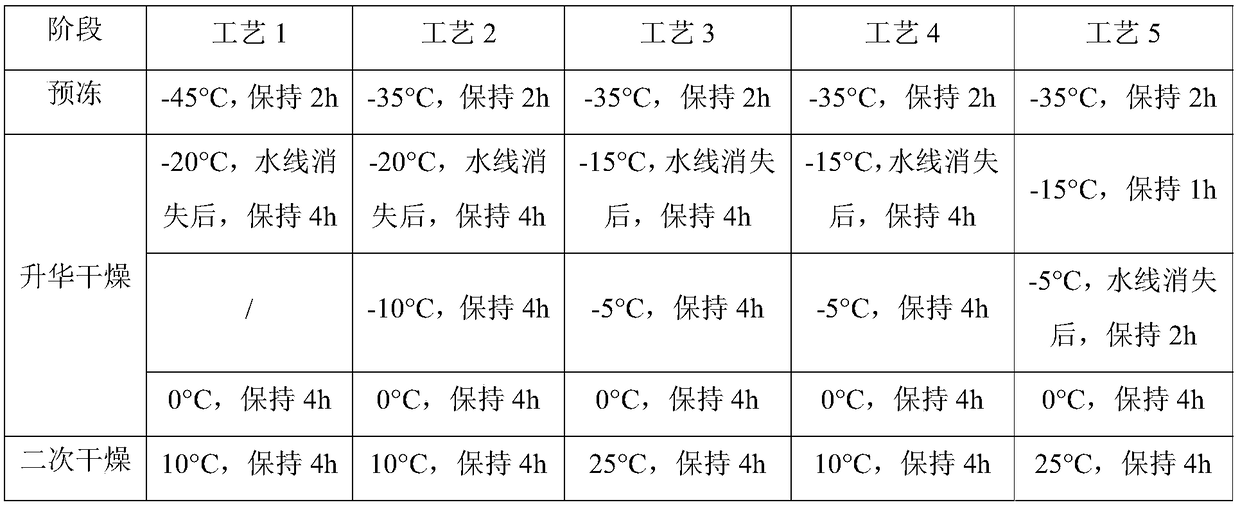
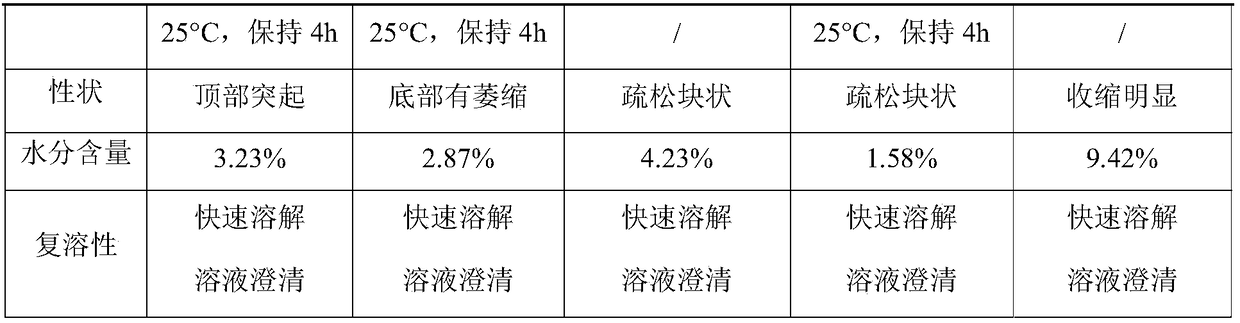
[0039] (3) Filling and freeze-drying in a freeze dryer.
[0040] (4) Freeze-drying: Pre-freeze the product to below -35°C and keep it for 2 hours; raise the temperature of the heat transfer oil to -15°C and keep it ...
Embodiment 2
[0041] Embodiment 2 Rat acute toxicity test
[0042]The quantitative determination of toxicity is to introduce different doses of the test substances into the experimental animals, and the dose sufficient to make 50% of the total number of individuals under the test conditions is called LD50 (half lethal dose), generally used per kilogram of body weight expressed in milligrams of toxicant.
[0043] Give test product dexlansoprazole for injection and reference substance lansoprazole for injection by disposable intravenous injection, observe the acute toxic reaction produced to tested animal SD rats, compare dexlansoprazole and lansoprazole The safety of Soprazole, toxicity test result are shown in Table 4 and Table 5.
[0044] The rat acute toxicity test result of table 4 dexlansoprazole
[0045] dose
250.0mg / kg
225.0mg / kg
202.5mg / kg
182.3mg / kg
164.0mg / kg
LD50
80%
60%
20%
10%
0%
221.5mg / kg
[0046]...
Embodiment 3
[0052] The tolerance of embodiment 3 Dexlansoprazole for injection
[0053] The tolerance test is a dose-escalation clinical tolerance test carried out in healthy subjects, the purpose is to observe the tolerance and safety of normal people after administration of dexlansoprazole for injection.
[0054] Subjects: healthy volunteers aged 18-45;
[0055] Drug source:
[0056] Dexlansoprazole for injection is prepared according to Example 1;
[0057] Lansoprazole for injection, purchased from Shandong Luoxin Pharmaceutical Co., Ltd., specification: 30mg, batch number: 513093217;
[0058] "For injection" means that the pharmaceutical dosage form is freeze-dried powder injection.
[0059] Drug preparation: constant-speed intravenous injection for 60 minutes, twice a day. When using this product, dilute it with 100mL of 0.9% Sodium Chloride Injection. Injection was filtered using an in-line filter with a pore size of 1.2 μm.
[0060] Clinical Observation Index
[0061] During...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com