A kind of β-mannosidase and its application
A technology of mannosidase and mannose, applied in the field of bioengineering, can solve problems such as limiting the application of β-mannosidase and difficult expression, and achieve the effect of potential industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Cloning of β-mannosidase LrMan5 gene
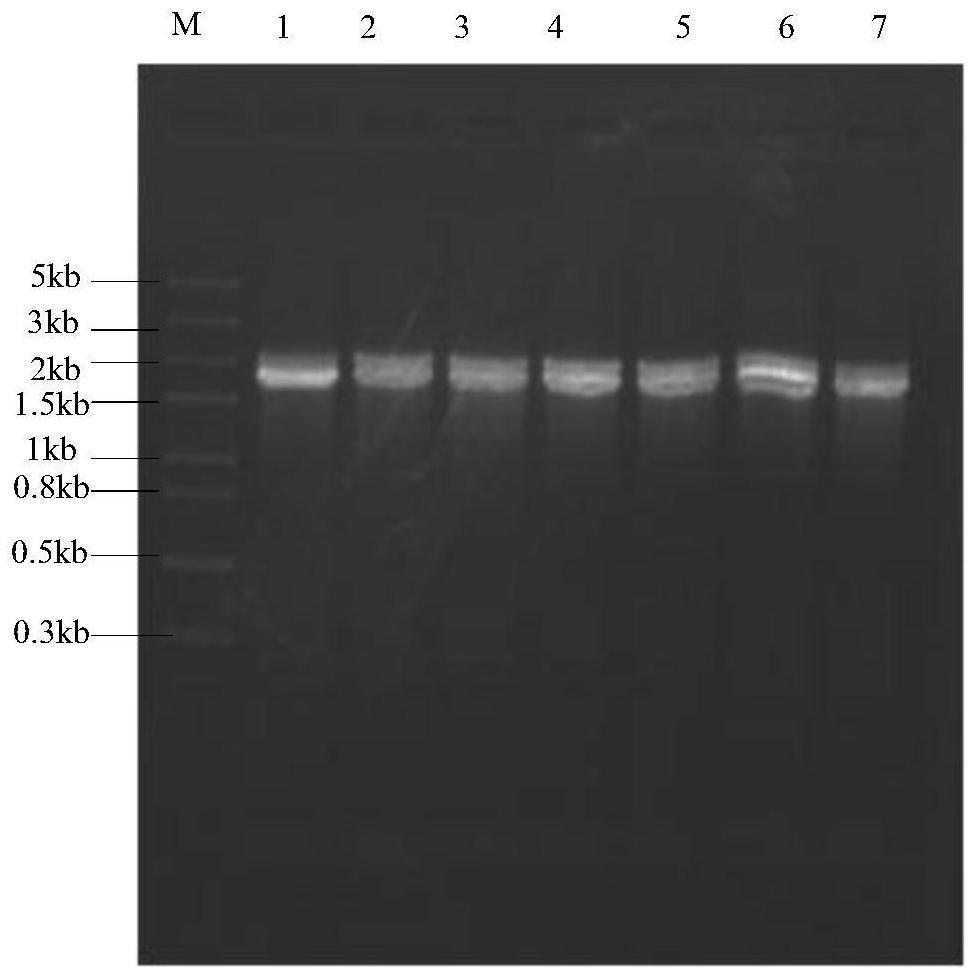
[0037] Extract the genomic DNA of Lichtheimia ramose, use the genomic DNA as a template, design primer LrMan5-F / R, the sequence is as shown in Table 1 (SEQ ID: 3 / SEQ ID: 4), obtain β-mannosidase by conventional PCR method (LrMan5) gene, the gene sequence is shown in SEQ ID NO:1.
[0038] Table 1: LrMan5 amplification primer sequences
[0039]
[0040] The PCR reaction system for obtaining the LrMan5 gene is shown in Table 2, and the reaction conditions are shown in Table 3.
[0041] Table 2: PCR reaction system
[0042]
[0043] Table 3: PCR reaction conditions
[0044]
Embodiment 2
[0045] Example 2 Construction and verification of Pichia pastoris expression vector comprising the gene encoding LrMan5
[0046] (1) LrMan5 gene and plasmid pPICZαA were double digested
[0047] Table 4 shows the double enzyme digestion reaction system of LrMan5 gene, and Table 5 shows the double enzyme digestion reaction system of plasmid pPICZαA.
[0048] Table 4: LrMan5 Gene Double Digestion Reaction System
[0049]
[0050] Table 5: Plasmid pPICZαA double enzyme digestion reaction system
[0051]
[0052] (2) Connection of pPICZαA vector and LrMan5 gene
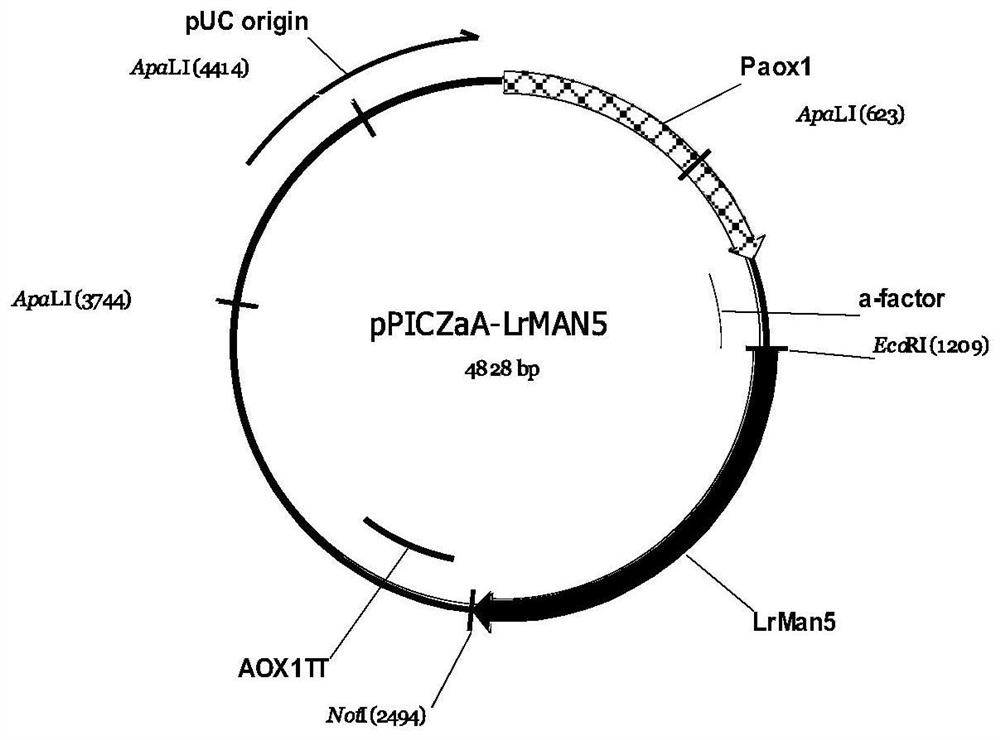
[0053] The connection system is shown in Table 6, and the expression vector pPICZαA-LrMan5 was constructed (the map of the expression vector is shown in figure 1 shown), transform Escherichia coli DH5a, and carry out vector screening and amplification.
[0054] Table 6: Connection system between pPICZαA vector and LrMan5
[0055]
[0056] (3) Conversion
Embodiment 3
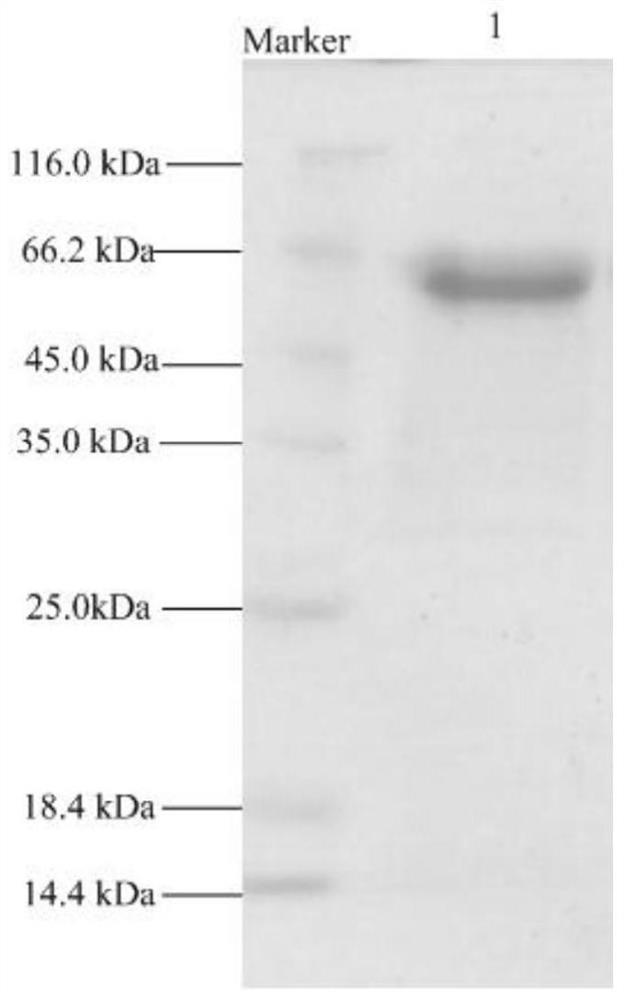
[0079] Example 3 Expression and purification of recombinant β-mannosidase LrMan5
[0080] A single colony of Pichia pastoris that grew well was picked, inoculated into a finger flask containing 5 mL of BMG, and cultured overnight at 30°C with shaking. Transfer the cultured bacterial solution overnight to a 500mL Erlenmeyer flask containing 50mL YPDG, seal it with eight layers of gauze to ensure a good air-permeable environment, and culture overnight at 28°C and 250rpm. Centrifuge the cultured bacterial solution overnight at 1500g for 3min, resuspend 50mL of fresh BMMY culture in a 500mL Erlenmeyer flask, seal with eight layers of gauze, and cultivate at 28°C with shaking at 250rpm. Methanol was added every 6h to a final concentration of 1.2-1.5% methanol. Take an appropriate amount of culture fluid every 12 hours to measure the OD600 value and enzyme activity, so as to determine the best harvest time for recombinant protein expression. The fermentation product was centrifuge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com