Preparation method of alkali metal difluorophosphate
A technology of difluorophosphoric acid base and hexafluorophosphoric acid base, applied in the direction of phosphorus halide/oxyhalide, etc., can solve problems such as high cost and unsafe process, and achieve the effects of reducing raw material cost, reducing cost and improving process safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
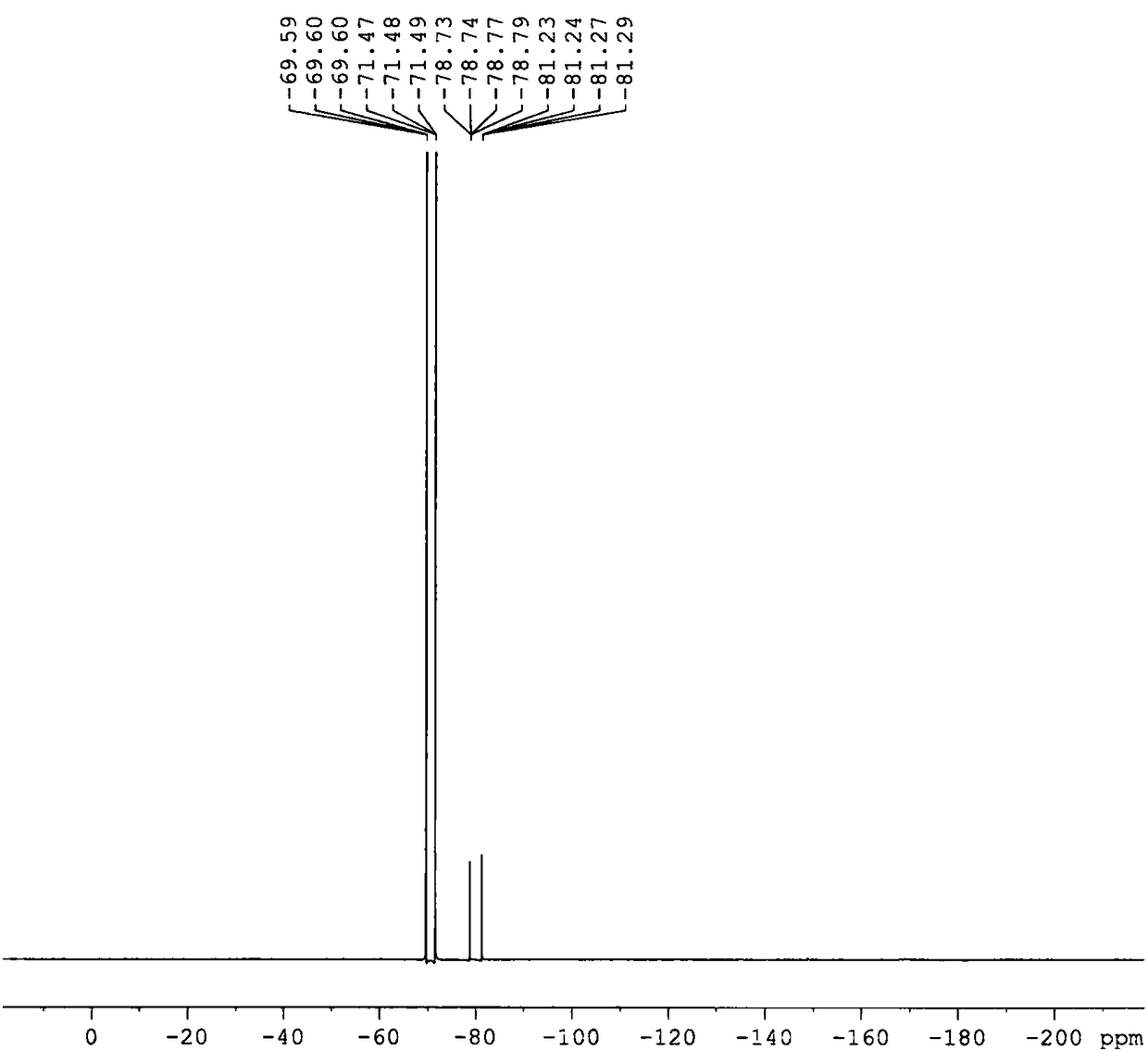
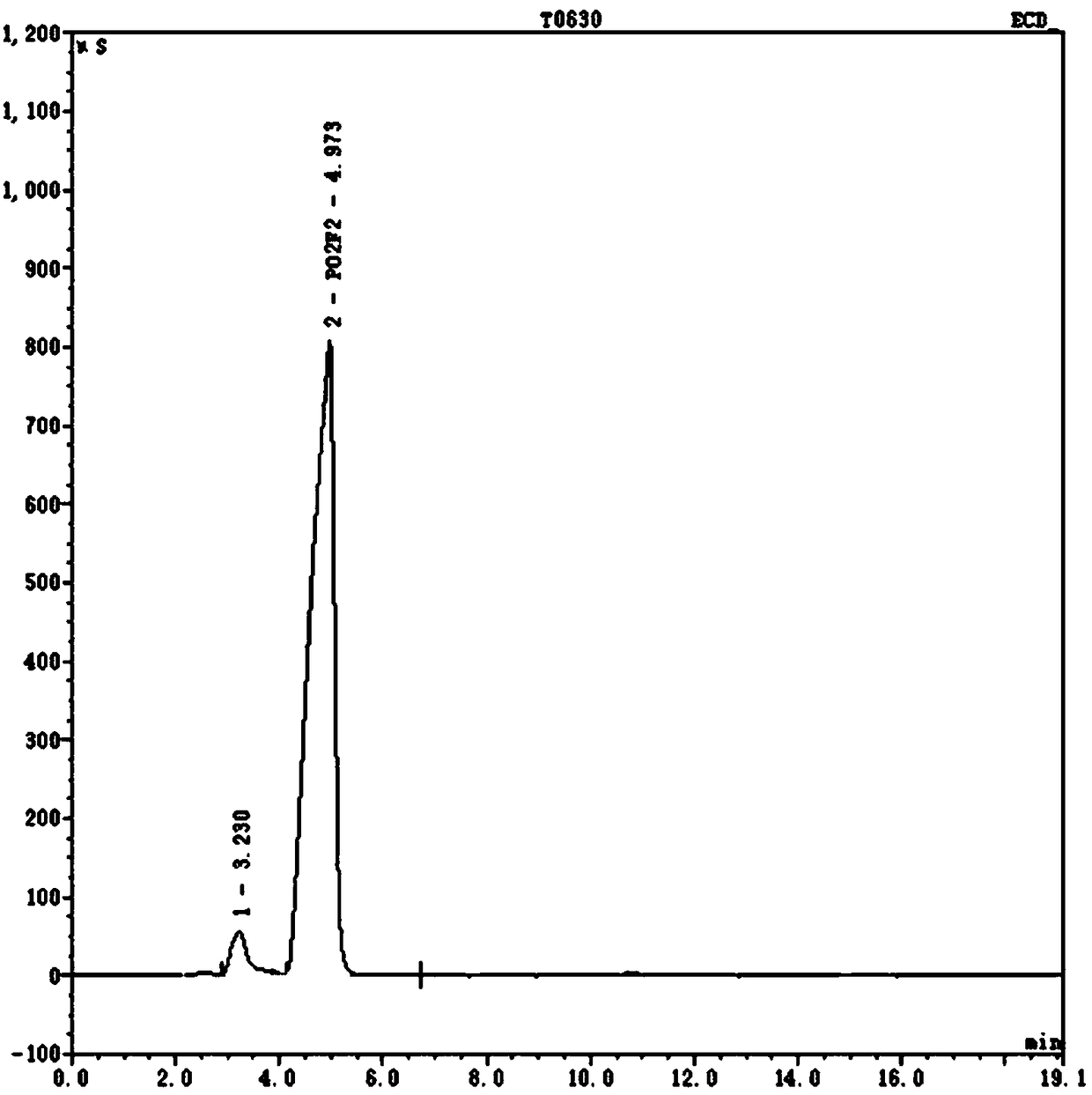
Image
Examples
example 1
[0036] Weigh 42.23g of battery-grade lithium hexafluorophosphate and 30.35g of lithium dihydrogen phosphate with a purity of 99.0% under argon environment protection, and add them to a 500ml jacketed three-neck flask, then add 300g of ethylene glycol dimethyl ether with a purity of 99.0%, and the upper part of the flask Connect the serpentine reflux condenser, and then connect the lye absorption device and the drying tower emptying device in sequence. The reaction was stirred at 80° C. for 6 hours, and naturally cooled to room temperature after the reaction. Filtrate under reduced pressure, take the filtrate and rotary evaporate, white crystals are gradually precipitated, and the obtained crystals are gradient vacuum-dried at 80°C to obtain the target product lithium difluorophosphate with a purity of 99.4%.
example 2
[0038]Weigh 33.87g of sodium hexafluorophosphate with a purity of 98.0% and 30.06g of sodium monohydrogen phosphate with a purity of 97.0% under the protection of argon gas and add them to a 200ml volume two-necked stainless steel reaction bottle, and place them on the rollers for mixing after initial mixing Machine mixing for 0.5 hours, so that the reaction raw materials are evenly mixed. React at a temperature of 100°C for 6 hours. During the reaction process, the stainless steel reaction bottle leads to the exhaust pipe, the lye absorption device, and the drying tower emptying device. After the reaction, cool naturally to room temperature, add the reaction solid to 300 g of ethylene glycol dimethyl ether extractant with a purity of 99.0%, stir for 1 hour and filter under reduced pressure, take the filtrate and concentrate it under reduced pressure, and with the volatilization of the extractant, crystals After continuous precipitation, the concentrated crystallization sample...
example 3
[0042] Weigh 35.19g of battery-grade lithium hexafluorophosphate and 25.30g of lithium dihydrogen phosphate with a purity of 99.0% under the protection of argon gas, and put them in a stainless steel reaction bottle connected with an alkali absorption device and a drying tower emptying device, and mix the materials with a roller machine After 0.5 hour, place it at 220° C. for 8 hours to react. Naturally cool to room temperature after the reaction is finished, use 300g of absolute ethanol with a purity of 99.7% as an extractant to dissolve the reaction mixture, stir and extract for 8 hours, so that the target product is extracted completely; then filter under reduced pressure, add an inert solvent after the filtrate is concentrated, and then After a solid-liquid separation operation, the obtained solid was transferred to a vacuum drying device, and dried by a gradient temperature raising method to obtain lithium difluorophosphate with a purity of 99.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com