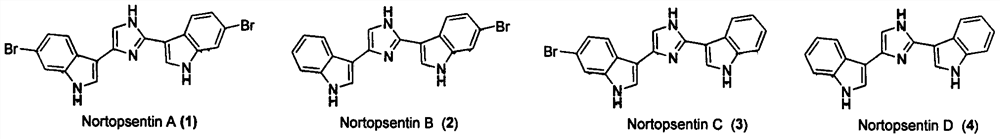
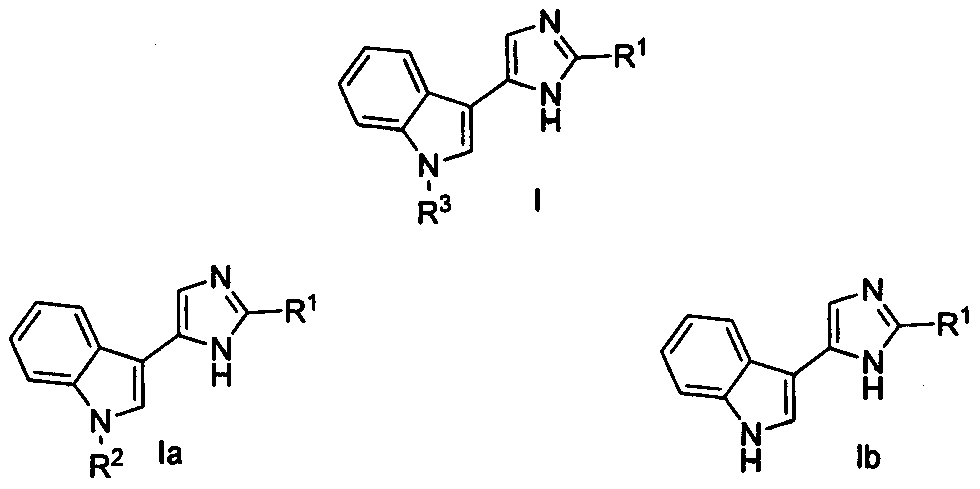
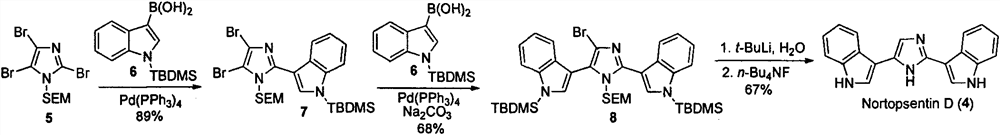
Nortopsentin alkaloid derivatives and their preparation and application in the prevention and treatment of diseases and insect pests
A technology of alkaloid derivatives and derivatives, which is applied in chemicals for biological control, botanical equipment and methods, applications, etc., can solve the problem of insufficient biological activity research, low natural content of Nortopsentin alkaloids, and difficult synthesis. and other problems, to achieve the effect of good insecticidal activity, good anti-plant virus and pathogen activity, and good insecticidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Synthesis of Nortopsentin alkaloid bisindole derivative structure Ia
[0035] 18: Add indole (3.51g, 30mmol) and 150mL acetonitrile to a 500mL single-necked bottle in sequence, add 60% NaH (1.44g, 42mmol) under ice-cooling, stir for about 10min, and add p-toluenesulfonyl chloride (6.27g, 33mmol), the addition was completed, and the reaction was resumed at room temperature. The reaction was monitored by TLC, and the reaction was completed in about 4 hours. with saturated NH 4 The Cl solution was quenched, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and precipitated to obtain 8.06 g of a brown solid with a yield of 99% and a melting point of 76-78°C. 1 H NMR (400MHz, CDCl 3 )δ7.99(d, J=8.4Hz, 1H), 7.76(d, J=8.4Hz, 2H), 7.56(d, J=3.6Hz, 1H), 7.52(d, J=7.6Hz, 1H) , 7.36-7.27(m, 1H), 7.24-7.18(m, 3H), 6.65(d, J=3.6Hz, 1H).
[0036] 19: Take a 500mL single-necked bottle, and put AlCl 3 (39.39g, 300mmol) was dissolved in DCM, and under ...
Embodiment 2
[0063] Embodiment 2: the assay of anti-tobacco mosaic virus activity, assay procedure is as follows:
[0064] 1. Virus purification and concentration determination:
[0065] Virus purification and concentration determination were carried out in accordance with the SOP specification for tobacco mosaic virus compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the crude virus extract was centrifuged twice with polyethylene glycol, the concentration was measured and refrigerated at 4°C for later use.
[0066] 2. Compound solution preparation:
[0067] After weighing, the original drug was dissolved in DMF to prepare 1×10 5μg / mL mother solution, and then diluted with 1‰ Tween 80 aqueous solution to the required concentration; Ningnanmycin preparation was directly diluted with water.
[0068] 3. In vitro effect:
[0069] Rub inoculation of leaves of Shanxi tobacco at the right age, rinse with running water, the virus concentration is 10 μg / ...
Embodiment 3
[0082] Embodiment 3: antibacterial activity test, assay procedure is as follows:
[0083] A. In vitro bactericidal test, bacterial growth rate determination method (plate method):
[0084] Dissolve a certain amount of medicine in an appropriate amount of acetone, then dilute it with an aqueous solution containing 200ug / mL emulsifier to the required concentration, then draw 1mL of the medicine solution into the petri dish, then add 9mL of medium, shake well and make 50ug / mL mL of the drug-containing plate, and a plate with 1 mL of sterilized water added as a blank control. Use a puncher with a diameter of 4mm to cut out the bacterial plate along the outer edge of the hyphae, and move it to the drug-containing plate. Each treatment was repeated three times. Place the culture dish in a constant temperature incubator at 24±1°C. After 48 hours, investigate the expanded diameters of the bacteria discs of each treatment, calculate the average value, and compare with the blank cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com