Polypeptide conjugates based on pillar aromatic hydrocarbons, their preparation methods and their applications
A pillar aromatic hydrocarbon and conjugate technology, which is applied in the field of pillar aromatic hydrocarbon-based polypeptide conjugates, can solve the problems of large toxic and side effects, limited therapeutic effect and application scope, non-targeting of chemotherapeutic drugs, etc. Problems, reaction conditions are mild and efficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
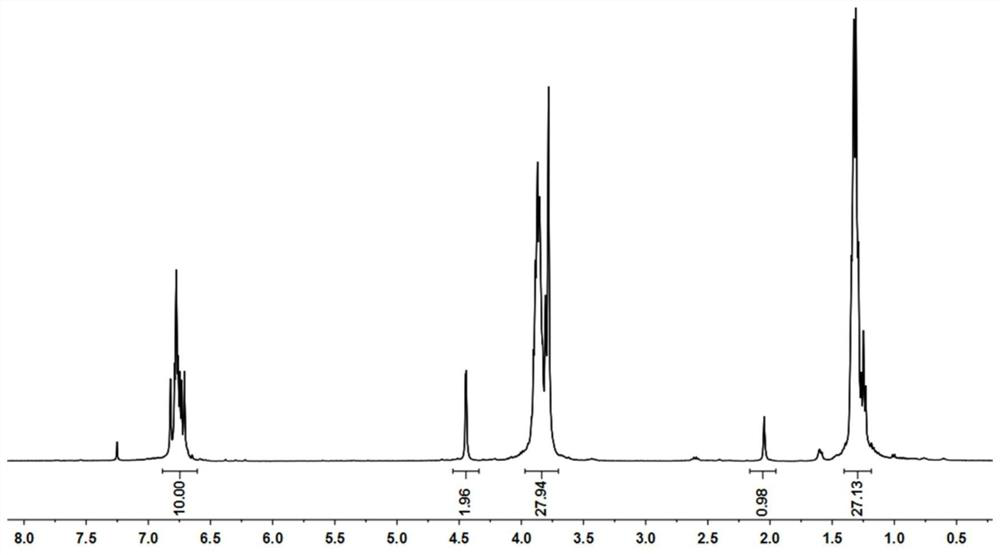
[0051] Example 1: Synthesis of column[5]arene-based polypeptide conjugates, see Figure 1 to Figure 5 ,
[0052] 1. Synthesis of ethoxy column [5] arene derivatives:
[0053]
[0054] Compound 2 was synthesized. To a solution of compound 1 (0.89 g, 1 mmol) in dichloromethane (50 ml) was added a solution of boron tribromide (0.0835 ml, 0.9 mmol) under nitrogen protection. Stir at room temperature for 4 h, quench the reaction with water, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, concentrate by distillation under reduced pressure, and pass through a column to obtain compound 2.
[0055] Compound 3 was synthesized. Under nitrogen protection, potassium carbonate (0.8 g, 5.8 mmol) was added to a solution of compound 2 (1 g, 1.16 mmol) in acetone (50 ml), stirred at 65°C under reflux, and 3-bromopropyne (0.154 ml, 1.89 mmol) was added dropwise. ) solution, dripped after about 10min, and continued stirring and refluxing for 24h. The reacti...
Embodiment 2
[0063] Example 2: Preparation and characterization of WCP5A / polyamine complexes
[0064] 1. Experimental samples
[0065] Due to the polypeptide conjugate P-EtP5A 1 The H-NMR spectrum is very complex, which is not conducive to the analysis of the host-guest interaction between them, so a water-soluble column [5] aromatic hydrocarbon (WCP5A) was selected as the research object.
[0066]
[0067] 2. Experimental method
[0068] Accurately weigh 21.25mg spermine (spm), 15.25mg spermidine (spd), 9.26mg (put) putrescine (0.105mmol), and mix with 171mg water-soluble column [5] aromatic hydrocarbon (WCP5A) (0.105mmol) respectively , dissolved in 5 mL of water, fully stirred, and then the solution of the mixture was vacuum freeze-dried to obtain a polyamine / WCP5A complex. The resulting complex, water-soluble column[5]arene (WCP5A) and three polyamines were dissolved in heavy water and subjected to NMR analysis.
[0069] 3. Experimental results
[0070] as below Image 6 , 7 a...
Embodiment 3
[0072] Example 3: Evaluation of the inhibitory effect of conjugate P-EtP5A on tumor cells
[0073] 1. Experimental samples
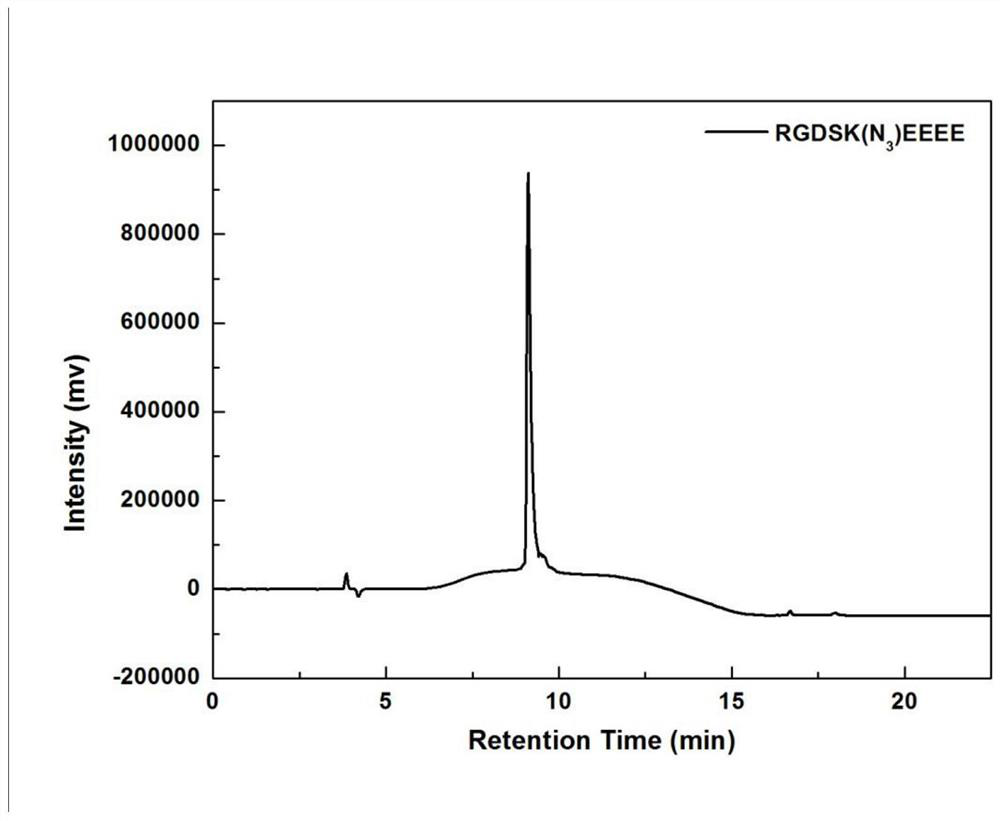
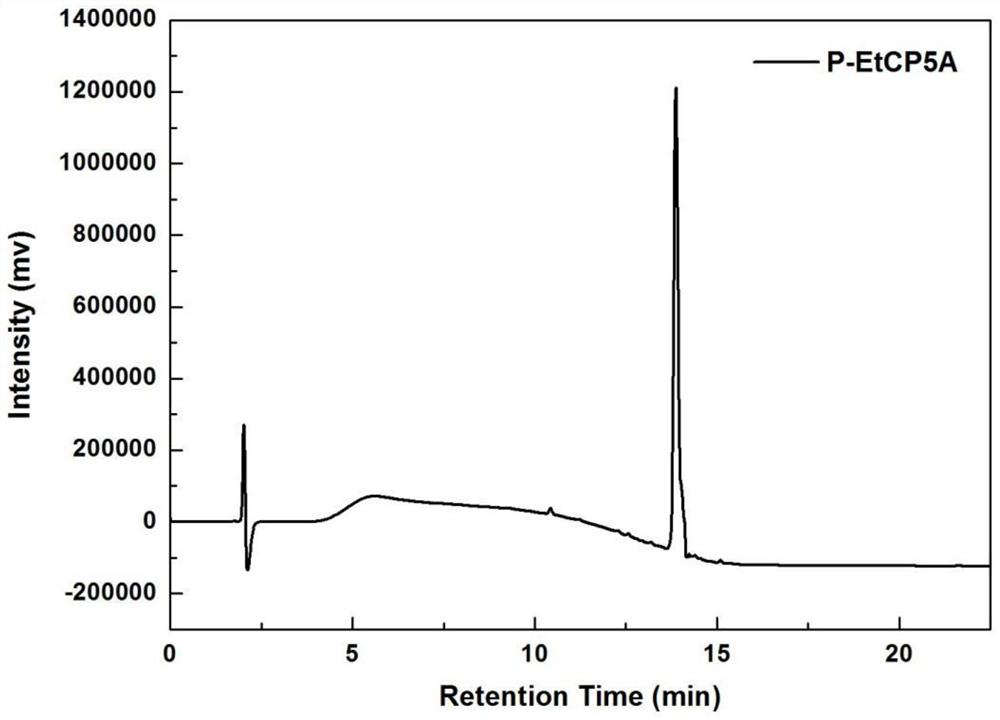
[0074] P-EtCP5A was prepared by the method of Example 1.
[0075] Breast cancer cells MCF-7 and liver cancer cells HepG2: both provided by Peking Union Medical College Cell Bank.
[0076] 2. Test method
[0077] DMEM medium (containing 10% FBS, 1% penicillin / streptomycin) for MCF-7 and 1640 medium (containing 10% FBS, 1% penicillin / streptomycin) for HepG2 in 5% CO 2 , cultured at a constant temperature of 37°C, and P-EtCP5A was dissolved in PBS to prepare a solution.
[0078] Collect the cells growing in log phase (MCF-7, HepG-2 cells), adjust the cell suspension concentration, inoculate the cell suspension in a 96-well plate, and plate to make the cell density to be tested to about 10,000 / well, 100 μL cells per well Suspension in 5% CO 2 , incubate at 37°C for 24h, observe the growth of cells adherently under the microscope, aspirate the medium in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com