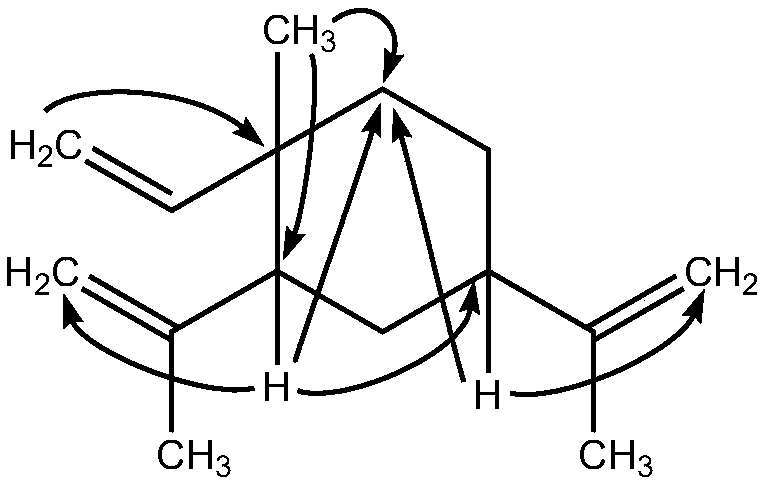
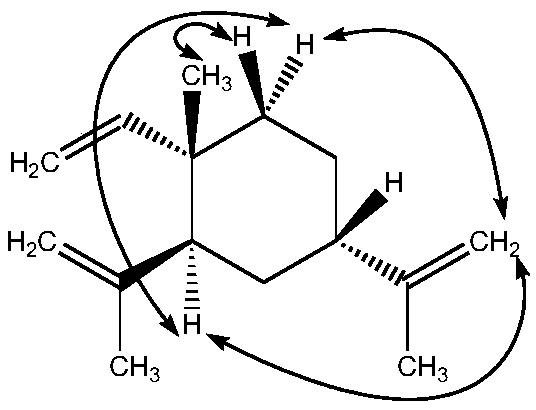
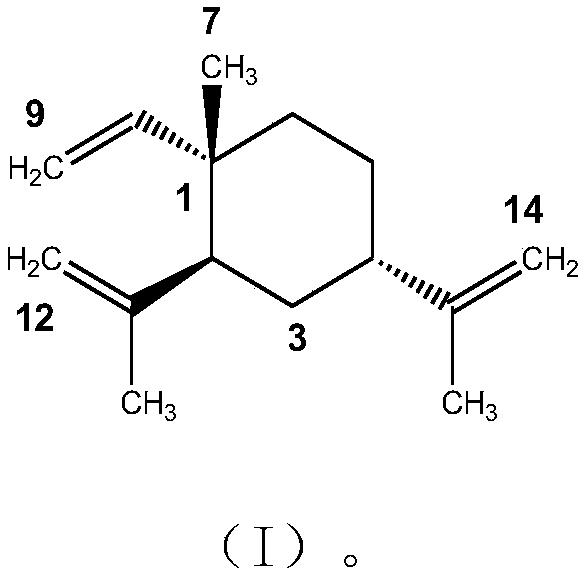
(1S, 2S, 4S)-beta-elemene and preparation method and application thereof
An elemene and vinyl technology, applied in the field of medicine, can solve the problem of not further reducing the side effects of β-elemene, β-elemene activity and the like, and achieve a quality control, anti-tumor activity and high safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1β-elemene stereoisomer 1R-methyl-1-vinyl-2R, the preparation of 4R-diisopropenyl cyclohexane
[0036] (1) Crude products containing β-elemene stereoisomers (from plant extracts containing β-elemene such as zedoary oil, citronella oil, and Eupatorium volatile oil, or from fermentation products obtained by yeast fermentation One or more), dissolved and loaded in n-hexane, separated by high performance preparative liquid chromatography, and eluted with a mixed solvent system of 90% acetonitrile and water as the mobile phase, flow rate: 15mL / min, detection wavelength: 210nm, chromatographic column: YMC-pack ODS-A 10μM, 20×250mm, at the peak time of about 17min, collect β-elemene and its stereoisomer 1R-methyl-1-vinyl-2R, 4R-diisopropenylcyclohexane 5.05g; (2) the sample obtained in step (1) is dissolved and loaded in n-hexane, and then separated by high-efficiency preparative liquid chromatography, with a mixed solvent system of 80% acetonitrile and water Eluti...
Embodiment 2
[0037] Example 2 Physical and chemical properties and structural identification of β-elemene stereoisomer 1R-methyl-1-vinyl-2R, 4R-diisopropenylcyclohexane
[0038] The β-elemene stereoisomer 1R-methyl-1-vinyl-2R,4R-diisopropenylcyclohexane prepared in Example 1 was used for physical and chemical property analysis.
[0039] Ultraviolet Spectrum: The maximum absorption wavelength of the β-elemene stereoisomer is 205nm.
[0040] Infrared spectrum: The β-elemene stereoisomers are at 3082, 2968, 2935, 2871, 1639, 1448, 1373, 889cm -1 moderate to strong peak intensity.
[0041] Specific rotation: Take the β-elemene stereoisomer, weigh it accurately, add absolute ethanol to dissolve, the concentration is 14mg / mL, and measure the specific rotation at 20°C to be 36.24.
[0042] Mass Spectrum: The high-resolution mass spectrum of the β-elemene stereoisomer shows that the molecular weight of the compound is [M] + =204.1877, molecular formula is C 15 h 24 .
[0043] NMR: 13 C NMR ...
experiment example 3
[0054] Experimental example 3 β-elemene stereoisomer 1R-methyl-1-vinyl-2R, 4R-diisopropenyl cyclohexane and β-elemene acute toxicity comparison
[0055] Samples tested: β-elemene stereoisomer 1R-methyl-1-vinyl-2R, 4R-diisopropenylcyclohexane, β-elemene, all produced by CSPC Yuanda (Dalian ) Pharmaceutical Co., Ltd., in liquid form, stored at 4°C.
[0056] Preparation method: Accurately weigh the sample, dissolve it with DMF, then add Tween 80 (TW80) to aid dissolution, and finally dissolve it in normal saline (NS), DMF:TW80:5%NS=10:2:88 (V:V: V), ultrasound for 5 minutes, ready to use.
[0057] Test animals: ICR mice, provided by Shanghai Lingchang Biotechnology Co., Ltd.; experimental animal quality certificate: SCXK (Shanghai) 2013-0016; certificate number: 2013001828818 2013001828481; experimental animal use license: SYXK (Su) 2012 -0004; body weight: 18-22g; number of animals: 100; sex: half male and half male.
[0058] Laboratory environment: room temperature 24±2°C, r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com