Method for continuously and efficiently preparing 2, 6, 6-trimethyl-2-cyclohexene-1, 4-diketone by oxidation
A cyclohexene, high-efficiency technology, applied in the field of synthesis of 2,6,6-trimethyl-2-cyclohexene-1,4-dione, can solve solvent entrainment loss and limited internal heat transfer area , Stirring speed is not fast and other problems, to achieve the effect of improving reaction efficiency and yield, shortening reaction residence time, and improving reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
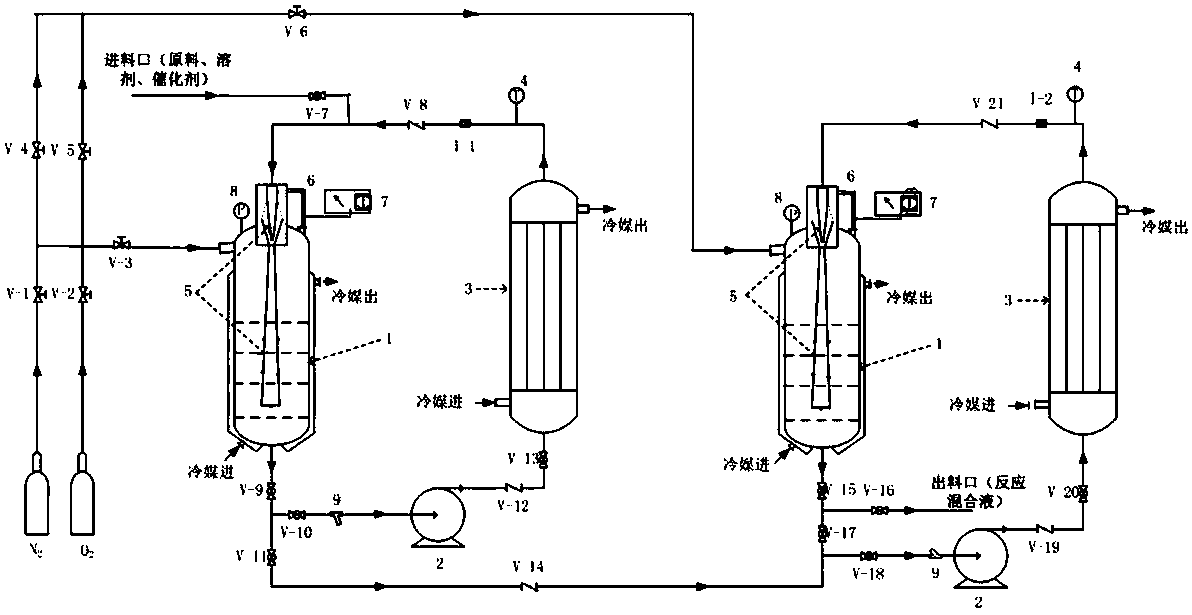
Image
Examples
Embodiment 1
[0049] According to the schematic flow chart, under the condition of continuous steady-state operation, will contain catalyst (iron acetylacetonate), solvent (triethylamine) and reaction raw material (3,5,5-trimethyl-3-cyclohexene-1- Ketone) mixed solution (mass ratio is 0.02:2:1) enters the two-tank series reaction system, the conditions and residence time are controlled to react until completion, the oxidation reaction solution is continuously discharged from the discharge port, and samples are taken for detection. Wherein the specific reaction parameters are as follows:
[0050] Reactor 1: oxygen content: 8%; pressure: 0.5MPA; liquid temperature in the reactor: 45°C; pump outlet temperature: 30°C; pump flow rate: 1m / s (equivalent to a jet velocity of 10m / s); residence time : 5h.
[0051] Reactor 2: Oxygen content is 6%, pressure: 0.4MPA, reactor liquid temperature: 60°C; pump outlet temperature: 45°C; pump flow rate: 1.2m / s (equivalent to jet velocity of 12m / s) stay Time:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com