Synthesis method of vortioxetine hydrobromide impurity
A technology of vortioxetine hydrobromide and its synthesis method, which is applied in the field of medicine and chemical industry, can solve the problems of few synthesis reports, etc., and achieve the effects of easy availability of raw materials, improved quality standards, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
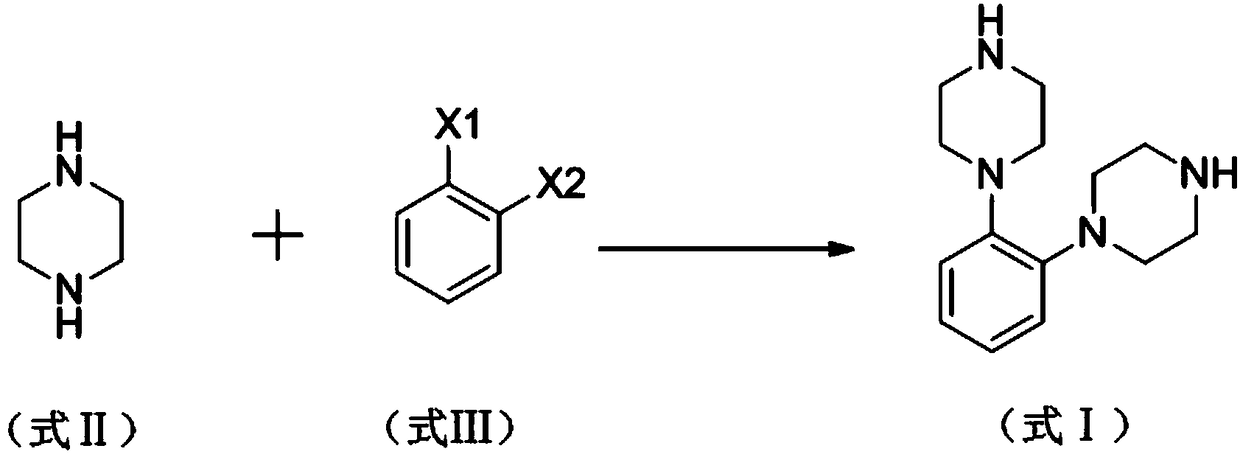
[0025] A kind of synthetic method of vortioxetine hydrobromide impurity proposed by the present invention, the steps are as follows:
[0026] In the 250ml there-necked flask, add 120mg (0.1mmol) tetrakis (triphenylphosphine) palladium, 7.15g triethylamine, 200ml toluene, logical N2 protection, stir at room temperature for 10min; 6.09g (70.7mmol) piperazine, 10g (35.3 mmol) o-bromoiodobenzene was dissolved in 20ml of toluene, added dropwise to a three-necked flask, under the protection of N2, heated and refluxed for 5h, cooled to room temperature, added 100ml of water, stirred for 15min, filtered, the filtrate was separated to remove the water layer, and 15% chlorine was added The sodium chloride solution was washed twice, and the organic layer was dried with anhydrous sodium sulfate and spin-dried.
[0027] Vortioxetine hydrobromide impurity 1,2-bis(piperazin-1-yl)benzene (I) was obtained by column chromatography, and the mobile phase was composed of petroleum ether and ethyl ...
Embodiment 2
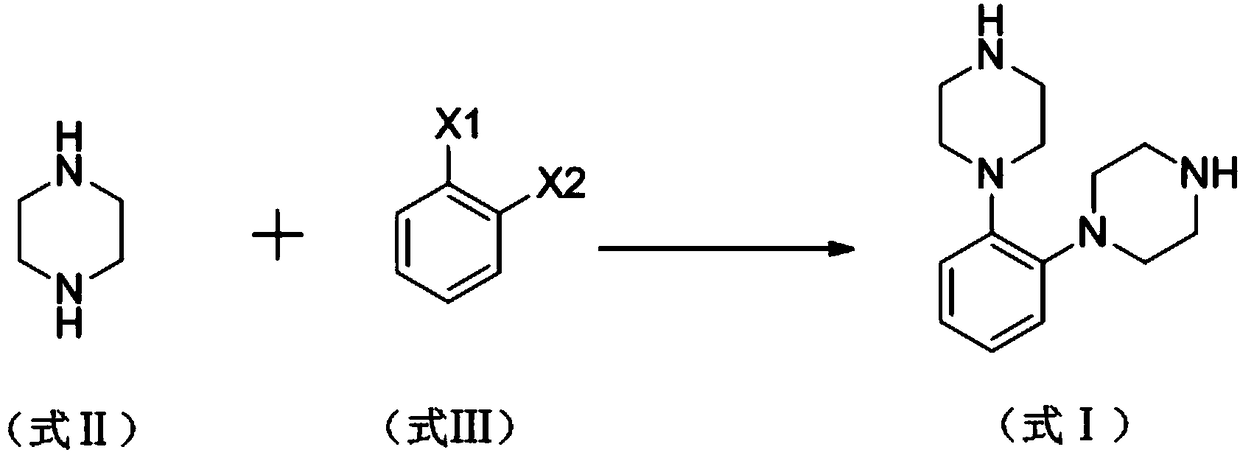
[0030] A kind of synthetic method of vortioxetine hydrobromide impurity proposed by the present invention, the steps are as follows:
[0031] Add 120mg (0.1mmol) tetrakis (triphenylphosphine) palladium, 16.9g sodium tert-butoxide, 200ml xylene to a 250ml three-necked flask, pass N2 protection, stir at room temperature for 10min; 12.1g (140.7mmol) piperazine, 10g (35.3mmol) o-bromoiodobenzene was dissolved in 20ml of xylene, added dropwise to a three-necked flask, under the protection of N2, heated and refluxed for 6h, cooled to room temperature, added 100ml of water, stirred for 15min, filtered, the filtrate was separated from the water layer, added Wash twice with 15% sodium chloride solution, dry the organic layer with anhydrous sodium sulfate, and spin dry.
[0032] Vortioxetine hydrobromide impurity 1,2-bis(piperazin-1-yl)benzene (I) was obtained by column chromatography, and the mobile phase was composed of petroleum ether and ethyl acetate at a volume ratio of 100:1.
...
Embodiment 3
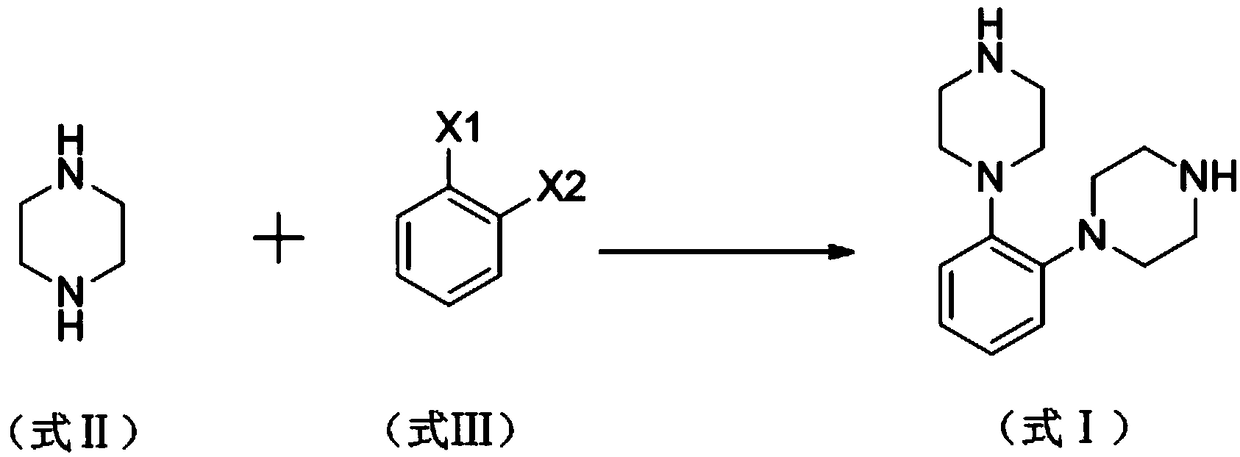
[0035] A kind of synthetic method of vortioxetine hydrobromide impurity proposed by the present invention, the steps are as follows:
[0036] Add 120mg (0.1mmol) tetrakis (triphenylphosphine) palladium to 250ml there-necked flask, 15.6g potassium carbonate, 200ml tetrahydrofuran, pass N2 protection, stir at room temperature for 10min; 6.86g (79.6mmol) piperazine, 10g (35.3mmol ) o-bromoiodobenzene was dissolved in 20ml of tetrahydrofuran, added dropwise to a three-necked flask, under the protection of N2, heated and refluxed for 8 hours, cooled to room temperature, added 100ml of water, stirred for 15 minutes, filtered, the filtrate was separated to remove the water layer, and 15% chlorinated The sodium solution was washed twice, and the organic layer was dried with anhydrous sodium sulfate and spin-dried.
[0037] Vortioxetine hydrobromide impurity 1,2-bis(piperazin-1-yl)benzene (I) was obtained by column chromatography, and the mobile phase was composed of petroleum ether an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com