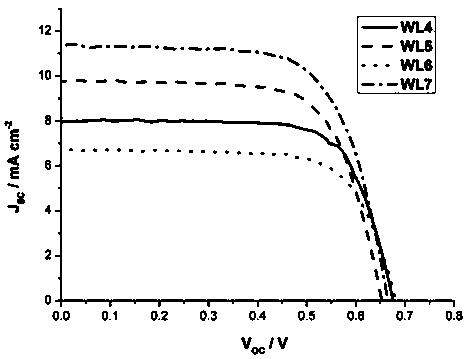
Triarylamine compound containing pyridazo quinazolone and preparation method and application thereof
A technology of pyridoquinazolinones and compounds, which is applied in the field of triarylamine compounds, can solve problems such as few reports, and achieve good photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
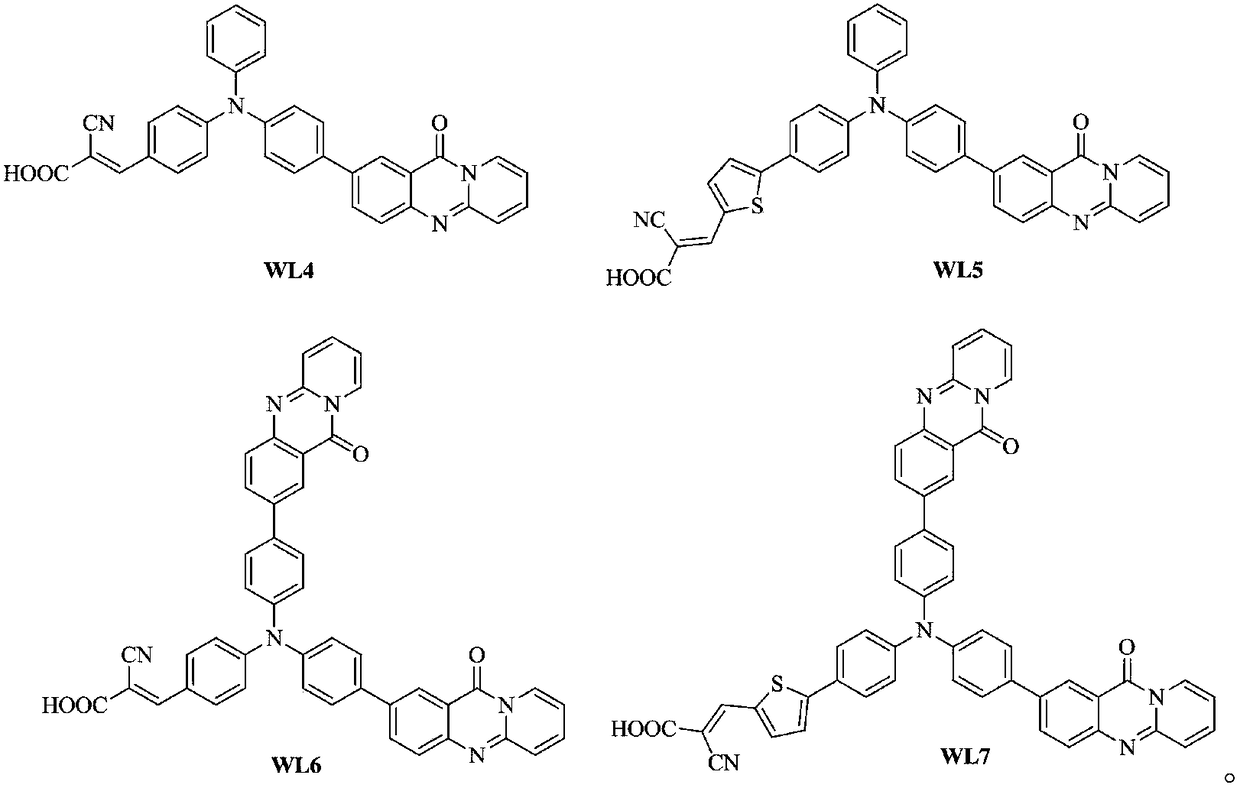
[0021] Synthesis of the compound represented by the formula (WL4) in Example 1:
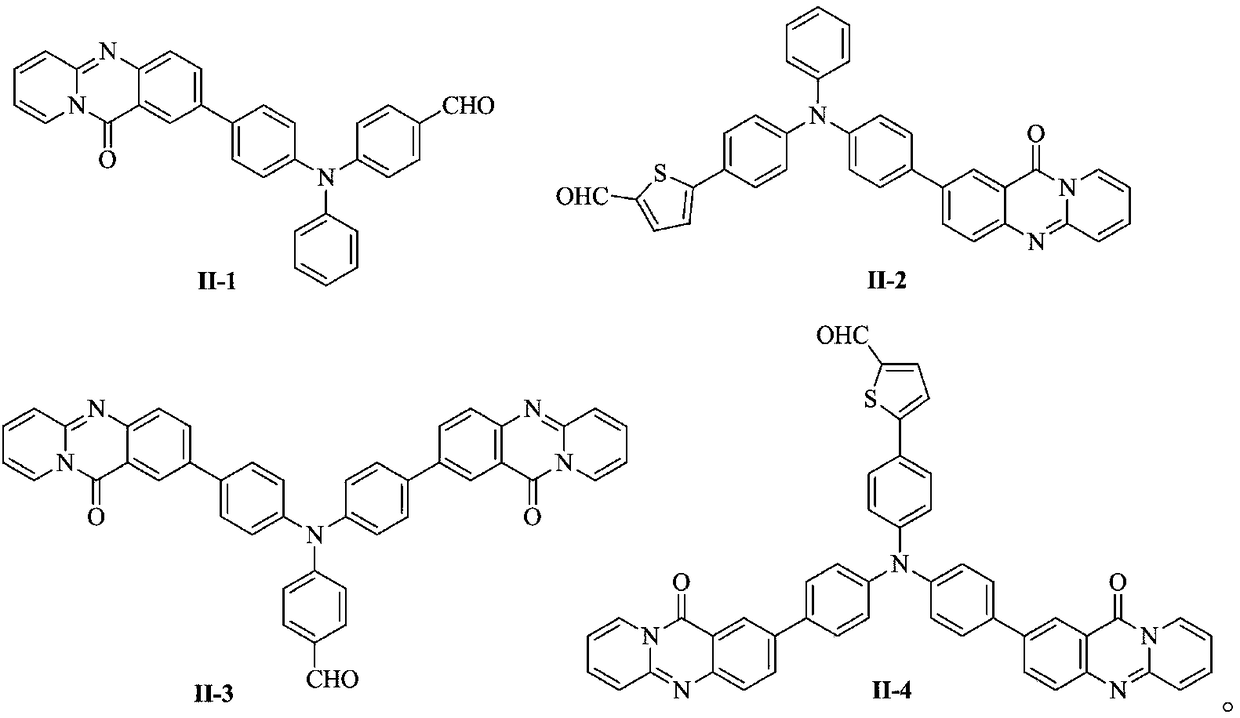
[0022] 1) Synthesis of compound represented by formula (II-1)
[0023] Under nitrogen protection, add 2-bromopyridoquinazolinone (0.275g, 1mmol), biboronic acid pinacol ester (0.253g, 1mmol), 1,1'-bis(diphenylphosphine) to the there-necked flask Ferrocene palladium chloride (the molecular formula is PdCl 2 (dppf), 0.022g, 0.03mmol), potassium acetate (0.294g, 3mmol) and 1,4-dioxane (15mL), heated and refluxed for stirring for 10h, after the reaction was completed, 100mL of water was added to the reaction solution, Then 30 mL of dichloromethane was added for extraction, left to stand for stratification, and the organic layer and the aqueous layer were obtained by liquid separation. The organic layer was washed with saturated brine and anhydrous Na 2 SO 4 Dry, then concentrate and evaporate the solvent to obtain the crude product I, which is directly used for the next reaction.
[0024] Under n...
Embodiment 2
[0032] The synthesis of compound shown in embodiment 2 formula (WL5):
[0033] 1) Synthesis of compound represented by formula (II-2)
[0034]Under nitrogen protection, compound of formula (IV) (0.434g, 1 mmol), potassium carbonate (0.276g, 2 mmol), tetrakistriphenylphosphine palladium (0.11 g, 0.1 mmol) and compound of formula (I) (0.32 g, 1 mmol) was dissolved in 50 mL of solvent (the solvent was V toluene: V methanol: V water=6:1:2), heated and refluxed for stirring for 10 h, after the reaction was completed, the reaction solution was cooled to room temperature, filtered, and the filtrate was evaporated The solvent, the residue after the solvent was evaporated was dissolved in dichloromethane, washed with saturated sodium chloride solution, anhydrous Na 2 SO 4 Drying, then concentrating and distilling off dichloromethane, the concentrate was separated by column chromatography to obtain 0.32 g of the compound represented by formula (II-2) as an orange powder (yield 58%), w...
Embodiment 3
[0041] The synthesis of compound shown in embodiment 3 formula (WL6):
[0042] 1) Synthesis of compound represented by formula (II-3)
[0043] Under nitrogen protection, add 2-bromopyridoquinazolinone (0.275g, 1mmol), biboronic acid pinacol ester (0.253g, 1mmol), PdCl to the there-necked flask 2 (dppf) (0.022g, 0.03mmol), potassium acetate (0.294g, 3mmol) and 1,4-dioxane (15mL), heated and refluxed for stirring for 10h, after the reaction was completed, 100mL of water and 30mL of dichloromethane, extracted, left to stand for stratification, separated to obtain an organic layer and an aqueous layer, the organic layer was washed with saturated brine in turn, anhydrous Na 2 SO 4 Dry, then concentrate and evaporate the solvent to obtain the crude product I, which is directly used for the next reaction.
[0044] Under nitrogen protection, crude product I (0.148 g, 0.4 mmol), compound represented by formula (V) (0.322 g, 1 mmol), Pd (PPh) 3 ) 4 (0.15g, 0.13mmol) and K 2 CO 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com