Application of ilexsaponin in preparation of anti-diabetic medicines
An anti-diabetic and hair-holly technology, applied in the field of medicine, can solve the problems of serious side effects and single target
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
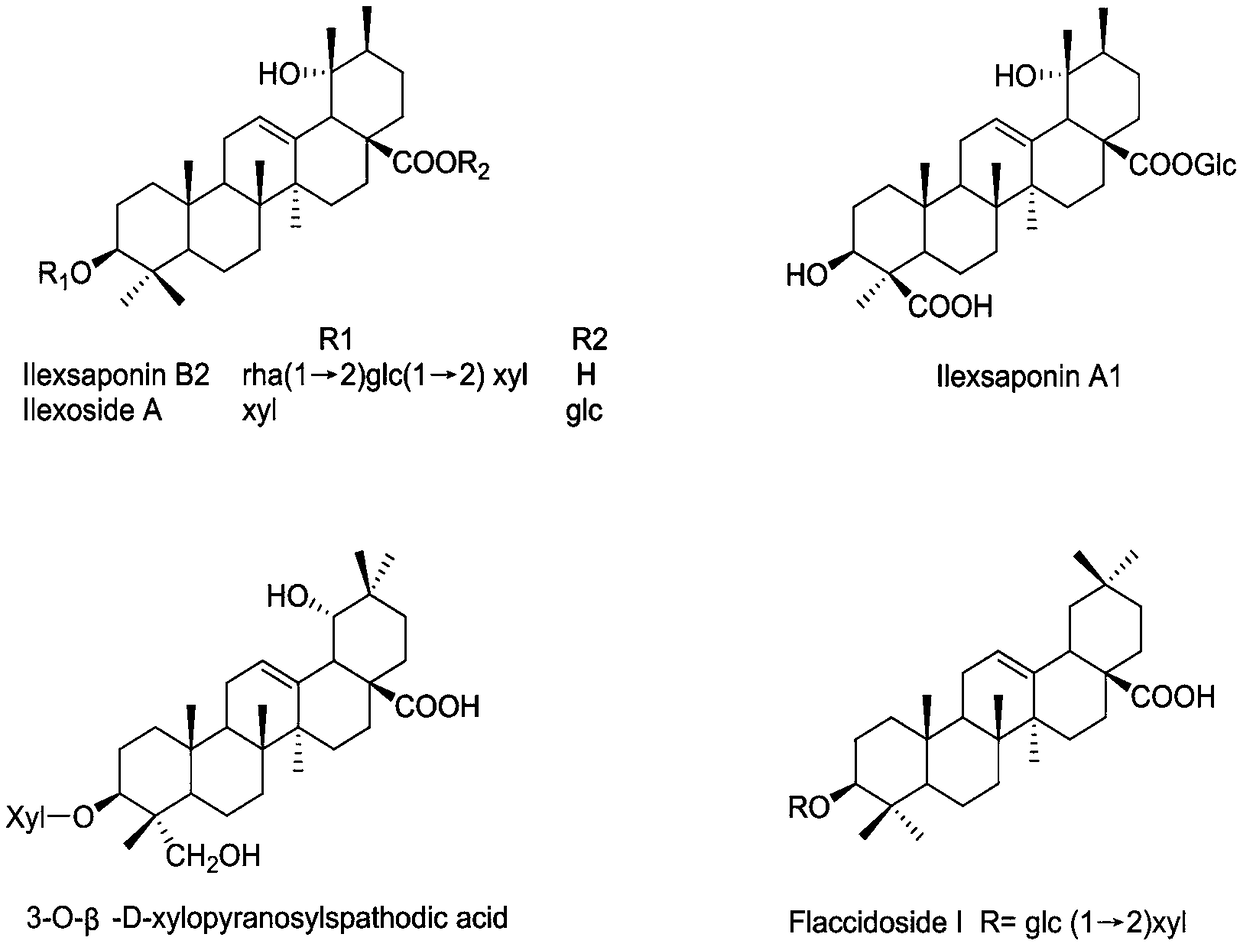
[0027] Example 1 Preparation of Ilex pubescens pentacyclic triterpene saponin 3-O-β-D-xylopyranosylspathodic acid and Flaccidoside I Weigh 10 kg of dry root of Ilex pubescens, add 70% ethanol to reflux and extract 3 times, 3h / time; combine the extracts, Concentrate under reduced pressure to obtain the total extract; weigh the total extract, add distilled water (2L / kg) to make a suspension, and extract with petroleum ether and n-butanol in turn to obtain the n-butanol extraction part; the n-butanol extraction part is decompressed Concentrate and dry in vacuum, dissolve in water and put on D101 macroporous resin column, carry out gradient elution with water, 30% methanol, 50% methanol and 95% methanol respectively, concentrate under reduced pressure and dry in vacuum to obtain 50% methanol elution site; take 50% methanol to wash Remove the site, mix the sample with silica gel (100-200 mesh), and then perform silica gel column chromatography (200-300 mesh), and then use dichlorome...
Embodiment 2
[0030] Example 2 Ilex pentacyclic triterpene saponin ilexoside A, ilexsaponin A1 and Ilexsaponin B 2 preparation of
[0031] Weigh 10 kg of dried root of Ilex pubescens, extract 3 times with 75% ethanol under reflux, 4h / time; combine the extracts, concentrate under reduced pressure to obtain the total extract; weigh the total extract, add distilled water (2L / kg) to make a suspension liquid, and then sequentially extracted with petroleum ether and n-butanol to obtain the n-butanol extraction part; the n-butanol extraction part was concentrated under reduced pressure and vacuum-dried, dissolved in water and placed on a macroporous resin column, and respectively water, 30% methanol, 50% methanol, Gradient elution with 95% methanol, concentration under reduced pressure and vacuum drying to obtain the elution site of 50% methanol; take the elution site of 50% methanol, mix the sample with silica gel (100-200 mesh), and then perform silica gel column chromatography (200-300 mesh), ...
Embodiment 3
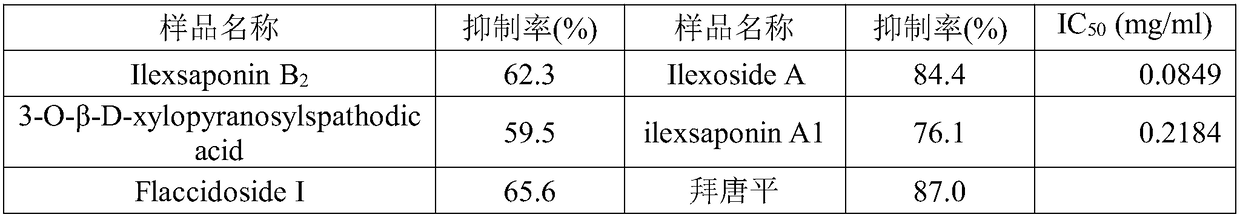
[0035] Example 3 α-glucosidase inhibitory activity screening
[0036] 1. Experimental materials
[0037] 1.1 Reagents
[0038] α-glucosidase (sigma); PNPG (sigma); disodium hydrogen phosphate, potassium dihydrogen phosphate, Nanjing Chemical Reagent Factory.
[0039] 1.2 Instruments
[0040] Pure water machine (Yipu Yida), ultrasonic instrument (Jiangsu Kunshan Ultrasonic Instrument Factory), microplate reader (Moleculardevices plus 384), pH meter (Mettler).
[0041] 2. Reagent preparation
[0042] α-glucosidase (sigma) was formulated with 0.067mol / L PH6.8 phosphate buffer solution to make 1U / mL, and the substrate PNPG (sigma) was made into 3mg / mL with water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com