Preparation process of phenolic hydroxy epoxidation product
A technology of oxidation products and phenolic hydroxyl rings, applied in chemical/physical processes, organic compound/hydride/coordination complex catalysts, catalytic reactions, etc., can solve environmental pollution, reduce hydrolyzable chlorine content, low epoxy products and other problems, to achieve process stability, reduce the content of hydrolyzable chlorine and inorganic chlorine, and reduce the effect of environmental protection pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]Heat 0.1 mol of benzyldiethylamine, 0.1 mol of γ-chloropropyltriethoxysilane, 100 mL of ethanol, and 0.02 mol of potassium iodide to 75°C under nitrogen protection, react for 1.5 hours, and obtain the quaternary ammonium salt mother liquor after filtering the product , Add 50mL of quaternary ammonium salt mother liquor dropwise to 100mL of distilled water / ethanol (20ml of water, 80ml of ethanol) to obtain a mixed solution, adjust the pH of the mixed solution to 5.0 with glacial acetic acid, and after standing for 2 hours, add 40 grams of halloysite nano The tube is put into it, fully soaked for 1-3 hours, and the water is removed by evaporation, and the obtained product is halloysite nanotube functionalized with quaternary ammonium salt.
[0049] Add bisphenol A and epichlorohydrin (molar ratio 1:2.1) under normal pressure, add the halloysite nanotube catalyst of the quaternary ammonium salt functionalization prepared in this embodiment (add catalyst according to benzyldi...
Embodiment 2
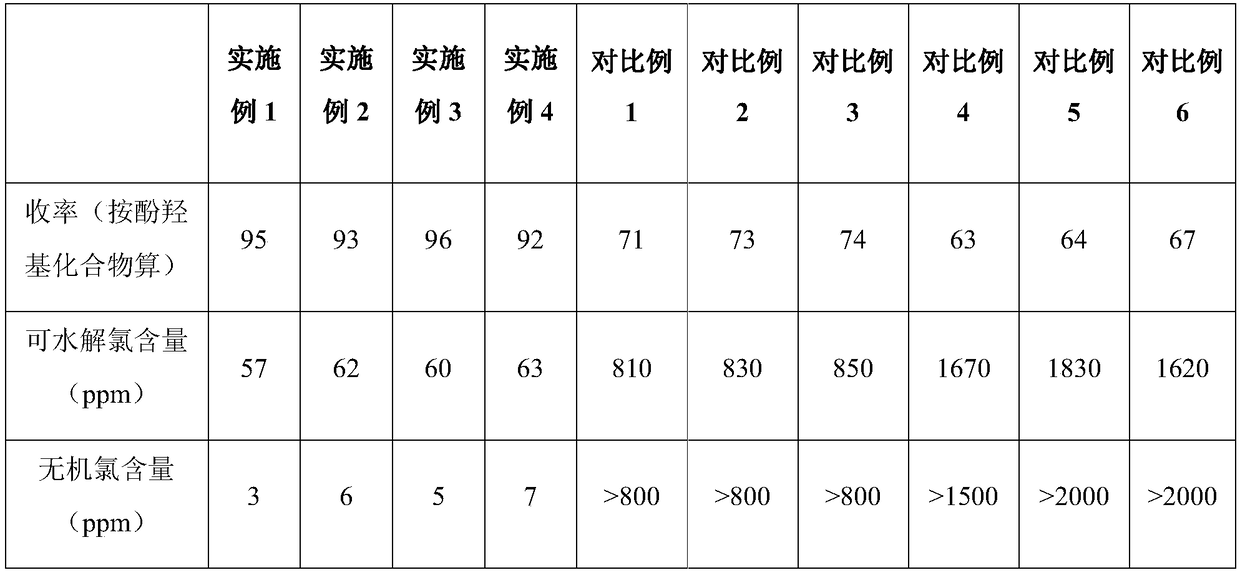
[0051] The raw materials and process parameters of the phenolic hydroxyl epoxidation process are exactly the same as in Example 1, except that the catalyst used is the halloysite nanotube catalyst that has been used 5 times and recovered in Example 1. The performance test results of the final product are listed in Table 1 below.
Embodiment 3
[0053] Heat 0.1 mol of benzyldiethylamine, 0.1 mol of γ-chloropropyltrimethoxysilane, 100 mL of methanol, and 0.02 mol of potassium iodide to 60°C under nitrogen protection, react for 1.5 hours, and filter the product to obtain a quaternary ammonium salt mother liquor. Add 50mL of quaternary ammonium salt mother liquor dropwise to 100mL of distilled water / methanol (20ml of water, 80ml of methanol), adjust the pH of this solution to 5.0 with glacial acetic acid, put 40g of halloysite nanotubes into it after standing for 2 hours, and fully After soaking for 3 hours, the water was removed by evaporation, and the obtained product was halloysite nanotubes functionalized with quaternary ammonium salts.
[0054] Add bisphenol F and epichlorohydrin (molar ratio 1:2) under normal pressure, add the halloysite nanotube catalyst of the quaternary ammonium salt functionalization prepared in this embodiment (add catalyst according to benzyldiethylamine raw material mol The amount is 0.005 o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com