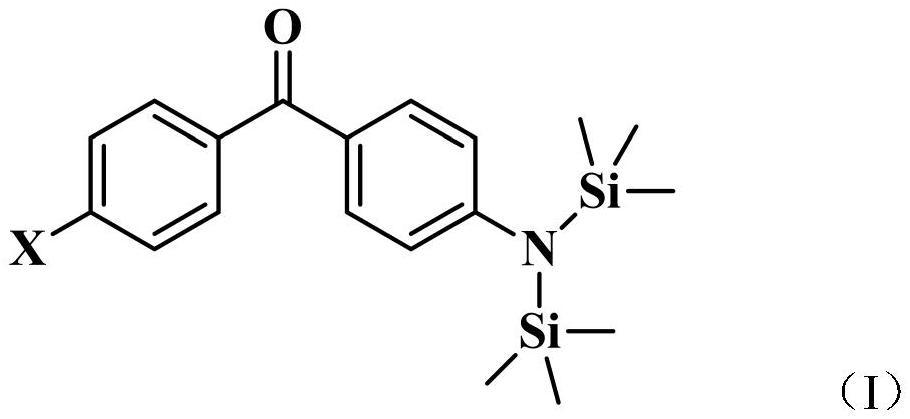
A kind of benzophenone derivative photoinitiator containing hexamethylsilylamine structure and preparation method thereof
A technology of hexamethylsilamine and benzophenone is applied in the field of preparation of benzophenone derivative photoinitiator photoinitiator, which can solve the problem of weak light intensity, low power conversion efficiency, influence on irradiation efficiency, etc. problem, to achieve the effect of high reaction rate, strong application value, and high photo-initiated activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
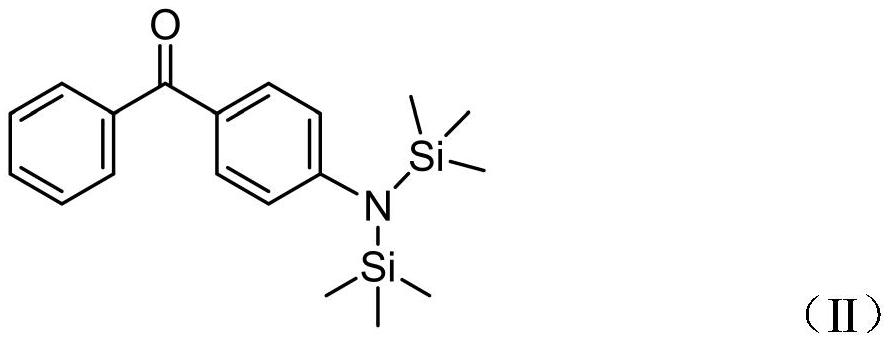
[0028] Using 80ml tetrahydrofuran solvent as the medium, add 3.0g (0.015mol) of 4-fluorobenzophenone and 2.75g (0.015mol) sodium bis(trimethylsilyl)amide, heat the reaction system to 80°C, and reflux for 4h; After the reaction was complete, the solid was filtered off, the solvent was spin-dried, heated and dissolved with 200ml of absolute ethanol, and cooled to crystallize to obtain 1.7g of a slightly yellow powder with a yield of 33.3% (theoretical yield 5.11g). The product 4-hexamethylsilylamine- The structure of benzophenone is shown in II, and the NMR data is: 1 H NMR (400MHz, DMSO): δ7.84(t, J=7.8Hz, 4H), 7.62(t, J=7.4Hz, 1H), 7.58–7.48(m, 4H), 0.04(s, 18H). The relative molecular mass in the mass spectrum ESI-MS normal spectrum is 341.1, confirming that the target product was synthesized.
[0029]
Embodiment 2
[0031] In 150ml of N,N-dimethylformamide (DMF) as a medium, add 6.5g (0.03mol) of 4,4-difluorobenzophenone and 5.68g (0.031mol) of bis(trimethylsilyl) Sodium amide, the reaction system was heated to 130°C, and refluxed for 6h; after the reaction was completed, the solid was filtered off, the solvent was spin-dried, heated and dissolved with 200ml of absolute ethanol, and 2.9g of slightly yellow powder was obtained by cooling and crystallization, with a yield of 27% (theoretical yield 10.77g), the structure of the product 4-hexamethylsilylamine-9-fluoro-benzophenone is shown in IV, and the NMR data are: 1 H NMR (400MHz, DMSO): 7.83–7.76(m,2H), 7.76–7.68(m,2H), 7.54(t,J=7.4Hz,2H), 7.13–7.06(m,2H), 0.02(s , 18H). Product M=359.15, Me 3 Si=73.05, mass spectrum ESI-MS (+) showed the following molecular ion peaks: 359.0 (65%), 287.0 (42%), confirming that the target product was synthesized.
[0032]
Embodiment 3
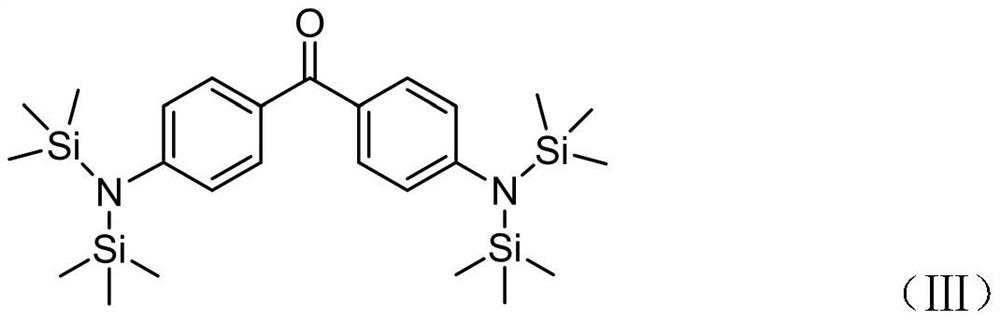
[0034]With 150ml of N,N-dimethylformamide (DMF) as the medium, add 6.5g (0.03mol) of 4,4-difluorobenzophenone and 12.81g (0.07mol) of bis(trimethylsilyl) Sodium amide, the reaction system was heated to 150°C, and refluxed for 6h; after the reaction was completed, the solid was filtered off, the solvent was spin-dried, heated and dissolved with 200ml of absolute ethanol, and cooled to crystallize to obtain 6.3g of slightly yellow powder with a yield of 42% (theoretical yield 15g), the structure of product 4,9-bis(hexamethylsilylamine)-benzophenone is as shown in (Ⅲ), and the NMR data are: 1 H NMR (400MHz, DMSO): 7.83–7.74(m,2H), 7.73–7.61(m,2H), 7.56(t,J=7.3Hz,2H), 7.11–7.03(m,2H), 0.05(s ,36H). Product M=500.5, Me 3 Si=73.05, mass spectrum ESI-MS (+) appeared the following molecular ion peaks: 500.5 (5%), 429.4 (15%), 428.3 (32%), 356.3 (100%), confirming that the target product was synthesized.
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com